Download In-Fusion™ Advantage PCR Cloning Kit User Manual
Transcript
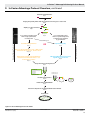
User Manual In-Fusion™ Advantage PCR Cloning Kit User Manual United States/Canada 800.662.2566 Asia Pacific +1.650.919.7300 Europe +33.(0)1.3904.6880 Japan +81.(0)77.543.6116 Clontech Laboratories, Inc. A Takara Bio Company 1290 Terra Bella Ave. Mountain View, CA 94043 Technical Support (US) E-mail: [email protected] www.clontech.com Cat. Nos. 639616, 639617, 639618, 639619, 639620, 639621, 639622, 639623 & 639624 PT4065-1 (PR9Z3431) Published January 2010 In-Fusion™ Advantage PCR Cloning Kit User Manual Table of Contents I. Introduction.............................................................................................................................. 3 II. In-Fusion Advantage Protocol Overview................................................................................ 4 III. List of Components................................................................................................................. 6 IV. Additional Materials Required................................................................................................ 7 V. PCR and Experimental Preparation........................................................................................ 8 A.Preparation of Linearized Vector by Restriction Digestion............................................................... 8 B. PCR Primer Design.............................................................................................................................. 8 C. PCR Amplification of Insert................................................................................................................11 D. Control Reactions...............................................................................................................................11 VI. Which Protocol Should You Follow?...................................................................................... 12 VII.Protocol I: In-Fusion Cloning Procedure w/Cloning Enhancer Treatment ........................ 12 A.Procedure for Treating Unpurified PCR Inserts with Cloning Enhancer ....................................... 12 B. In-Fusion Cloning Procedure for Cloning Enhancer-Treated PCR Inserts...................................... 12 VIII.Protocol II: In-Fusion Cloning Procedure w/Spin-Column Purification............................. 14 A.Procedure for Spin-Column Purification of PCR Inserts................................................................. 14 B. In-Fusion Cloning Procedure for Spin Column-Purified PCR Inserts............................................. 14 IX. Transformation Procedure..................................................................................................... 15 X. Expected Results.................................................................................................................... 15 XI. Troubleshooting Guide.......................................................................................................... 16 List of Figures Figure 1. The In-Fusion Cloning Method.........................................................................................................3 Figure 2. In-Fusion Advantage Protocol Flowchart........................................................................................5 Figure 3. Universal primer design for the In-Fusion System........................................................................9 Figure 4. Examples of primers designed for In-Fusion cloning..................................................................10 List of Tables Table I. In-Fusion Advantage Protocol Outline...............................................................................................4 Table II. Recommended Amount of Vector per In-Fusion Reaction............................................................12 Table III. Recommended Amount of Cloning Enhancer-Treated Insert per In-Fusion Reaction................13 Table IV. Recommended In-Fusion Reactions for Purified Inserts............................................................. 14 Table V. Troubleshooting Guide for In-Fusion Experiments.........................................................................16 Contact Us For Assistance Customer Service/Ordering: Technical Support: Telephone: 800.662.2566 (toll-free) Telephone: 800.662.2566 (toll-free) Fax: 800.424.1350 (toll-free) Fax: 650.424.1064 Web: www.clontech.com Web: www.clontech.com E-mail: [email protected] E-mail: [email protected] Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 2 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual I. Introduction In-Fusion™ Advantage PCR Cloning Kits are designed to join multiple pieces of DNA that have 15 bases of homology at their linear ends. A typical use for this technology would be to clone PCR products into vectors, without the use of restriction enzymes, ligase or phosphatase. InFusion cloning kits, which contain our proprietary In-Fusion Enzyme, let you rapidly generate very precise constructs. In-Fusion is high-throughput-compatible and universal—it works with any insert and any vector. 30 min single-tube reaction x x Recombinant vector Amplify your gene of interest PCR product Design gene-specific primers with 15 bp extensions homologous to vector ends Any linearized vector The In-Fusion Enzyme creates single-stranded regions at the ends of the vector and PCR product, which are then fused due to the 15 bp homology Figure 1. The In-Fusion Cloning Method. The In-Fusion Advantage PCR Cloning Method The In-Fusion method is simple and efficient. First, PCR primers are designed that share 15 bases of homology with the sequence at the ends of the linearized cloning vector (i.e., at the desired site of insertion; refer to Section V of this manual). These primers are then used to PCR amplify the insert DNA. The resulting PCR product is treated with our proprietary Cloning Enhancer or spincolumn purified, and combined with the linearized vector in the In-Fusion cloning reaction. In general, the In-Fusion reaction consists of a simple 30 minute incubation of the PCR product with the linearized cloning vector, followed by transformation into E. coli (Figure 1). Each reaction generates precise constructs with correctly oriented inserts and no additional nucleotides. The procedure is quite simple, so it is easily automated. With many vectors, such as our pDNR-Dual (and the linearized pUC19 control vector provided with this Kit), blue/white selection on X-Gal plates can be used to screen out rare non-linearized vector background. Although the highest cloning efficiency is achieved with a high-quality PCR product that appears on an agarose gel as a single, dense band of DNA (with minimal background), PCR products that contain additional non-specific background can also be cloned using In-Fusion. In such cases, the target PCR product is not treated with the Cloning Enhancer. It is, instead, isolated by gel extraction and spin column-purified before cloning. Figure 2 illustrates the differences in the experimental workflow required for Cloning Enhancer treated PCR products versus those that are spin column-purified. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 3 In-Fusion™ Advantage PCR Cloning Kit User Manual II. In-Fusion Advantage Protocol Overview The table below is a general outline of the protocol used in the In-Fusion Advantage PCR Cloning Kits. This outline is further illustrated in Figure 2. Please refer to the specified pages for details on performing each step. Step Table I. In-Fusion Advantage Protocol Outline Action 1 Select a base vector and identify the insertion site. Linearize the vector by restriction enzyme digestion or inverse PCR and purify. 2 Design PCR primers for your gene of interest with 15 bp extensions (5’) that are homologous to the ends of the linearized vector. 8-10 3 Amplify your gene of interest with a high-fidelity DNA polymerase. 11 4 Verify on an agarose gel that your target DNA has been amplified and determine the integrity of the PCR product. If a single prominent band of desired size is obtained, you can EITHER treat your insert with Cloning Enhancer (follow Protocol I), OR treat your insert with DpnI and spin-column purify (follow Protocol II). If a non-specific background smear or multiple bands are visible on your gel, isolate your target fragment by gel extraction and spincolumn purify (follow Protocol II). 11-12 5 Treat your target fragment with Cloning Enhancer OR spin-column purify. Cloning Enhancer Protocol I (p.12) OR Spin-Column Protocol II (p.14) 6 Determine the appropriate amount of Cloning Enhancer-Treated or Cloning Enhancer Protocol I (p.12) Spin Column-Purified PCR product (insert) and vector to use in your OR In-Fusion cloning reaction. Spin-Column Protocol II (p.14) 7 Set up your In-Fusion cloning reaction: 2 μl of 5X In-Fusion Reaction Buffer 1 μl of In-Fusion Enzyme X μl of Vector X μl of Insert X μl of dH20 to a Total Reaction Volume of 10 μl. Mix well. Cloning Enhancer Protocol I (p.13) OR Spin-Column Protocol II (p.14) 8 Incubate the reaction for 15 min at 37°C, followed by 15 min at 50°C, then place on ice. Cloning Enhancer Protocol I (p.13) OR Spin-Column Protocol II (p.14) 9 Bring the reaction volume up to 50 µl with TE buffer (pH 8), and mix well. Cloning Enhancer Protocol I (p.13) OR Spin-Column Protocol II (p.14) 10 Transform competent cells with 2.5 μl of the diluted reaction mixture from Step 9. Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 4 Pages 8 15 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual II. In-Fusion Advantage Protocol Overview, continued Generate a linearized vector Step 1 Design gene-specific primers with 15 bp extensions homologous to vector ends Step 2 15 bp M 1 2 Amplify your gene of interest M 1 2 Step 3 15 bp PCR product If you obtain pure PCR product with a single specific band, no background If you obtain PCR product with non-specific background, isolate the target fragment by gel extraction Step 4 Treat with DpnI Spin-Column purify OR Step 5 Step 5 Spin-Column Protocol II Cloning Enhancer Protocol I Treat with Cloning Enhancer Add 2 µl of Cloning Enhancer to 5 µl of PCR product and incubate 15 min at 37º C, 15 min at 80º C Step 5.2 Determine the volume of Cloning Enhancertreated insert and linearized vector to use in the In-Fusion reaction Mix the purified PCR insert and vector together at a 2:1 molar ratio Step 6 Step 6 Set up the In-Fusion cloning reaction Step 7 2 μl 5X Buffer 1 μl In-Fusion Enzyme x μl Vector x μl Insert x μl dH2O 15 min at 37º C 15 min at 50º C 10 μl Total Volume Incubate cloning reaction Step 8 x x Recombinant vector Dilute reaction with TE Buffer Step 9 Transform competent E. coli with the diluted reaction mixture Step 10 Screen clones Figure 2. In-Fusion Advantage Protocol Flowchart Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 5 In-Fusion™ Advantage PCR Cloning Kit User Manual III. List of Components The In-Fusion Advantage PCR Cloning Kits are available in 10, 50 and 100 reaction sizes, with or without Cloning Enhancer or NucleoSpin® Extract II. Store NucleoSpin Extract II at room temperature. Store all other components at –20°C. In-Fusion™ Advantage PCR Cloning Kits Components Cat. Nos. 639619 639620 639621 Rxns. 10 rxns 50 rxns 100 rxns 10 μl 50 μl 100 μl 20 μl 100 μl 200 μl 5 μl 5 μl 5 μl 10 μl 10 μl 10 μl In-Fusion Enzyme 5X In-Fusion Reaction Buffer pUC19 Control Vector,* linearized (50 ng/μl) Component Amounts 2 kb Control Insert (40 ng/μl) In-Fusion™ Advantage PCR Cloning Kits w/NucleoSpin® Components Cat. Nos. 639622 639623 639624 Rxns. 10 rxns 50 rxns 100 rxns In-Fusion Enzyme 10 μl 50 μl 100 μl 5X In-Fusion Reaction Buffer 20 μl 100 μl 200 μl 5 μl 5 μl 5 μl 2 kb Control Insert (40 ng/μl) 10 μl 10 μl 10 μl NucleoSpin Extract II 10 preps 50 preps 100 preps pUC19 Control Vector,* linearized (50 ng/μl) Component Amounts In-Fusion™ Advantage PCR Cloning Kits w/Cloning Enhancer Components Cat. Nos. 639616 639617 639618 Rxns. 10 rxns 50 rxns 100 rxns In-Fusion Enzyme 10 μl 50 μl 100 μl 5X In-Fusion Reaction Buffer 20 μl 100 μl 200 μl 5 μl 5 μl 5 μl 2 kb Control Insert (40 ng/μl) 10 μl 10 μl 10 μl Cloning Enhancer 50 μl 100 μl 200 μl pUC19 Control Vector,* linearized (50 ng/μl) Component Amounts *These Kits contain only enough vector for the control reactions. pUC19 Control Vector Information is available on line at www.clontech.com/support/vectors.asp Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 6 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual IV. Additional Materials Required The following materials are required but not supplied: • TE Buffer (pH 8.0) required for diluting the In-Fusion reaction prior to transformation 10 mM Tris-HCl 1 mM EDTA • Sodium Acetate (3 M) required only if concentrating DNA by precipitation • Glycogen (20 µg/µl) required only if concentrating DNA by precipitation • Ampicillin (100 mg/ml stock) or other antibiotic required for plating the In-Fusion reaction • LB (Luria-Bertani) medium (pH 7.0) • LB/antibiotic plates • SOC medium 2% 0.5% 10 mM 2.5mM 10 mM 20 mM Tryptone Yeast Extract NaCl KCl MgCl2•6H2O glucose 1. For 1 liter, dissolve 20 g of tryptone, 5 g of yeast extract, and 0.5 g of NaCl in 950 ml of deionized H2O. 2. Prepare a 250 mM KCl solution by dissolving 1.86 g of KCl in deionized H2O for a total volume of 100 ml. Add 10 ml of this stock KCl solution to the solution prepared in Step 1. 3. Adjust pH to 7.0 with 5 M NaOH, then bring the volume to 980 ml with deionized H2O. 4. Prepare a 1 M solution of MgCl2 by dissolving 20.33 g of MgCl2•6H2O in deionized H2O for a total volume of 100 ml. 5. Autoclave both solutions on liquid cycle at 15 lbs/in2 for 20 min. 6. Meanwhile, make a 2 M solution of glucose by dissolving 36 g of glucose in deionized H2O for a total volume of 100 ml. Filter-sterilize this solution. 7. Let the autoclaved solutions cool to about 55°C, then add 10 ml of the filter-sterilized 2 M glucose solution and 10 ml of 1 M MgCl2. Store at room temperature or 4°C. • Competent Cells We recommend the use of Fusion-Blue™ Competent Cells or any commercially-available competent cells (e.g., DH10B™, DH5α™) that have a transformation efficiency ≥ 1.0 x 108 cfu/µg. Fusion-Blue Competent Cells are available in 24-transformation (Cat. No. 636700) and 96-transformation (Cat. No. 636758) formats. • Cloning Enhancer (Cat. Nos. 639613, 639614 & 639615) [Optional] Cloning Enhancer is provided with some of the In-Fusion Advantage PCR Cloning Kits and can also be purchased separately. Cloning Enhancer removes background template DNA and PCR residue, eliminating the need for PCR insert purification prior to cloning when a single PCR product (i.e., no background) is obtained (See Section V.C.). • Spin Columns—NucleoSpin® Extract II (Cat. Nos. 740609.10, 740609.50 & 740609.250) [Optional] Spin columns can be used to purify PCR products, eliminating the need for gel extraction when a single PCR product (i.e., no background) is obtained (See Section V.C.). However, Spin Columns can also be used in conjunction with gel purification (e.g., if non-specific background or multiple bands are visible on an agarose gel). When spin columns are needed, we recommend NucleoSpin® Extract II. NucleoSpin® Extract II is provided with some of the In-Fusion Advantage PCR Cloning Kits and can also be purchased separately. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 7 In-Fusion™ Advantage PCR Cloning Kit User Manual V. PCR and Experimental Preparation PLEASE READ ENTIRE PROTOCOL BEFORE STARTING. A. Preparation of Linearized Vector by Restriction Digestion To achieve a successful In-Fusion reaction, you must first generate a linearized vector (with a very low background of uncut vector present). The linearized vector can be generated using restriction enzymes (single or double digests) or by PCR. Due to differences in cutting efficiencies, different restriction enzymes will generate different amounts of background. Generally speaking, two enzymes cut better than any single enzyme. Efficiency of digestion will always be better if the restriction sites are as far apart as possible. In addition, increasing the enzyme digestion time and the digestion reaction volume will reduce the background. Prepare a linearized vector by restriction enzyme digestion as follows. 1. We recommend cutting the vector with two different enzymes to reduce background, unless there is only one site available for cloning. 2. Incubate your restriction digest as directed by the restriction enzyme supplier. For many enzymes, incubation from 3 hours to overnight can increase linearization and reduce background. 3. After digestion, purify the linearized vector using any available PCR purification kit. We recommend using the NucleoSpin® Extract II Kit. 4. [Control] Check the background of your vector by transforming 5–10 ng of the linearized and purified vector into competent cells (See Transformation Procedure, Section IX). If the background is high, continue digesting the vector for a longer time after the addition of more restriction enzyme(s). Incubate 2 hours to overnight. Gel purify the remainder of the vector and transform again. B.PCR Primer Design Primer design and quality are critical for the success of the In-Fusion reaction. In-Fusion allows you to join two or more fragments, e.g. vector and insert (or multiple inserts), as long as they share 15 bases of homology at each end. Therefore, In-Fusion PCR primers must be designed in such a way that they generate PCR products containing ends that are homologous to those of the vector. Figure 3 outlines the guidelines for primer design and Figure 4 gives specific examples of In-Fusion PCR primers. When designing In-Fusion PCR primers, consider the following: 1. Every In-Fusion primer must have two characteristics: The 5’ end of the primer must contain 15 bases that are homologous to 15 bases at one end of the DNA fragment to which it will be joined (i.e., the vector or another insert). The 3’ end of the primer must contain sequence that is specific to the target gene. 2. The 3’ portion of each primer should: • be gene-specific. • be between 18–25 bases in length, and have a GC-content between 40–60%. • have a melting temperature (Tm) between 58–65°C. The Tm difference between the forward and reverse primers should be ≤ 4°C, or you will not get good amplification. Note: The Tm should be calculated based upon the 3’ (gene-specific) end of the primer, and NOT the entire primer. If the calculated Tm is too low, increase the length of the gene-specific portion of the primer until you reach a Tm of between 58–65°C. • not contain identical runs of nucleotides.The last five nucleotides at the 3’ end of each primer should contain no more than two guanines (G) or cytosines (C). Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 8 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual V. PCR and Experimental Preparation, continued 3. Avoid complementarity within each primer to prevent hairpin structures, and between primer pairs to avoid primer dimers. 4. You can perform a BLAST search to determine if the 3’ portion of each primer is unique and specific (at www.ncbi.nlm.nih.gov/BLAST/). 5. Clontech provides an online tool (at http://bioinfo.clontech.com/infusion/) that simplifies In-Fusion PCR primer design for standard cloning reactions. Simply provide your vector sequence, the restriction enzyme(s) used to linearize the vector (if that is the chosen method for linearization), and the primer sequence required to amplify your region of interest. 6. We generally use desalted oligonucleotide primers in PCR reactions. However, primer quality can depend on the vendor and varies from lot to lot. If your primer quality is particularly poor (i.e., has many premature termination products), or your primers are longer than 45 nucleotides, they may need to be PAGE purified; however, we usually find this is unnecessary. Forward Primer NNNNNNNNNNNNNNN Linearized Vectors with 5' Overhangs NNNNNNNNNNNNNN 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 append with your specific sequence * 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 * NNNNNNNNNNNNNN NNNNNNNNNNNNNNN Reverse Primer Forward Primer NNNNNNNNNNNNNNN Linearized Vectors with Blunt ends 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 NNNNNNNNNNNNNNNNNN 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 * * NNNNNNNNNNNNNNNNNN NNNNNNNNNNNNNNN Reverse Primer Forward Primer NNNNNNNNNNNNNNN Linearized Vectors with 3' Overhangs NNNNNNNNNNNNNNNNNNNNNN 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 * 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 * NNNNNNNNNNNNNNNNNNNNNN NNNNNNNNNNNNNNN Reverse Primer Guidelines for universal primer design To determine the 15 b homology sequence to be incorporated into each primer, start at the 5’ end of each DNA strand in the linearized vector (*). The region of homology for a particular primer consists of bases that are complementary to the first 15 bases at the 5’ end of a particular DNA strand. This means that the bases complementary to 5' overhangs are included in the primer sequence, but the bases in 3’ overhangs are not. Brackets indicate bases to be included in the 15 b region of homology Figure 3. Universal primer design for the In-Fusion System. Successful insertion of a PCR fragment requires that the PCR insert share 15 bases of homology with the ends of the linearized vector. This sequence homology is added to the insert through the PCR primers. For vectors with sticky ends, bases complementary to 5’ overhangs are included in the primer sequence; bases in the 3’ overhangs are not. See Figure 4 for specific examples. An online tool is also provided to assist in primer design and can be found at http://bioinfo.clontech.com/infusion/. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 9 In-Fusion™ Advantage PCR Cloning Kit User Manual V. PCR and Experimental Preparation, continued 5' Forward Primer SalI 5'-G AAG TTA TCA GTC GAC XXX XX...-3' A 5'-...ATA CAT TAT ACG AAG TTA TCA G 3'-...TAT GTA ATA TGC TTC AAT AGT CAG CT pDNR-CMV sequence AGC TTT CTA GAC CAT TCG TTT GGC G...-3' AA GAT CTG GTA AGC AAA CCG C...-5' 3'-...X XXX XXT TCG AAA GAT CTG GTA-5' HindIII 3' Reverse Primer SmaI 5'-ACC GGA CAT ATG CCC GGG XXX...-3' B 5'-...TCA GTC GAC GGT ACC GGA CAT ATG CCC 3'-...AGT CAG CTG CCA TGG CCT GTA TAC GGG GGG AAT TCC TGC AGG ATC CGC T...-3' CCC TTA AGG ACG TCC TAG GCG A...-5' 3'-...XXX GGG CCC TTA AGG ACG TCC-5' SmaI KpnI 5'-AG TTA TCA GTC GAC GGT ACC XXX...-3' C 5'-...CAT TAT ACG AAG TTA TCA GTC GAC GGT AC 3'-...GTA ATA TGC TTC AAT AGT CAG CTG C C GGA CAT ATG CCC GGG AAT T...-3' CA TGG CCT GTA TAC GGG CCC TTA A...-5' 3'-...XXX CCA TGG CCT GTA TAC GGG CC-5' KpnI Figure 4. Examples of primers designed for In-Fusion cloning. The above figure shows examples of primers designed with recognition sites for restriction enzymes that generate: 5’ overhangs (Panel A), blunt ends (Panel B), and 3’ overhangs (Panel C). The primer sequences are shown in bold. The Xs represent bases corresponding to the gene or sequence of interest. Additional nucleotides (indicated with a black box) have been added to each primer in order to reconstruct the restriction sites. They are not part of the 15 bases of sequence homology. Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 10 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual V. PCR and Experimental Preparation, continued C. PCR Amplification of Insert For most DNA polymerases, 10–100 ng of plasmid DNA is typically enough to use as a PCR template. However, if you are amplifying from a pool of cDNA, the amount of template DNA required depends on the relative abundance of the target message in your mRNA population. The In-Fusion method is not affected by the presence or absence of A-overhangs, so you can use any thermostable DNA polymerase for amplification, including proofreading enzymes. For the best results, we recommend using our Advantage® HD Polymerase Mix (Cat. No. 639241), which offers high-fidelity, efficient amplification of long gene segments (>1 kb), and automatic hot start for increased specificity and reduced background. For high yields and error-free amplification of inserts up to 5 kb, we recommend using the Advantage® HF 2 enzyme supplied in our Advantage HF 2 PCR Kits (Cat. Nos. 639123 & 639124). If you will be performing PCR with Advantage HD Polymerase, we recommend using the following amounts of template (for a 50 µl reaction): Human Genomic DNA E. coli Genomic DNA λ DNA Plasmid DNA 5 ng–200 ng 100 pg–100 ng 10 pg–10 ng 10 pg–1 ng If you choose not to use Advantage HD, we recommend that you use a robust, high fidelity, thermostable DNA polymerase that is capable of hot start PCR. When PCR cycling is complete, analyze your PCR product by agarose gel electrophoresis to confirm that you have obtained a single DNA fragment and to estimate the concentration of your PCR product. Quantify the amount of DNA by measuring against a known standard or DNA mass ladder ladder run on the same gel. Attention IMPORTANT: Following PCR, verify on an agarose gel that your target fragment has been amplified. If a single band of desired size is obtained, you can EITHER treat your PCR product with Cloning Enhancer (follow Protocol I) OR treat your PCR product with DpnI and spin-column purify (follow Protocol II). However, if non-specific background or multiple bands are visible on your gel, we recommend that you isolate your target fragment by gel extraction, then spin-column purify (follow Protocol II). D. Control Reactions When using the In-Fusion kit for the first time, we strongly recommend that you perform the positive and negative control reactions in parallel with your In-Fusion cloning reaction. Performing the control reactions will verify that the system is working properly. The 2 kb Control Insert included in the In-Fusion Advantage PCR Cloning Kits has already been purified, so there is no need for further treatment prior to the cloning reaction. To perform the control reactions, proceed with the In-Fusion Cloning Procedure for Spin Column-Purified PCR Inserts (Section VIII.B). Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 11 In-Fusion™ Advantage PCR Cloning Kit User Manual VI. Which Protocol Should You Follow? Following PCR, verify by agarose gel electrophoresis that your target fragment has been amplified. If a single band of the desired size is obtained, you can EITHER treat your PCR product with Cloning Enhancer (follow Protocol I), OR treat your PCR product with DpnI and spin-column purify (follow Protocol II). However, if nonspecific background or multiple bands are visible on your gel, we recommend that you isolate your target fragment by gel extraction, then spin-column purify (follow Protocol II). VII.Protocol I: In-Fusion Cloning Procedure w/Cloning Enhancer Treatment A. Procedure for Treating Unpurified PCR Inserts with Cloning Enhancer Attention IMPORTANT: DO NOT treat purified PCR products with the Cloning Enhancer. Before setting up the In-Fusion cloning reaction, treat unpurified PCR products (e.g. inserts) as follows: 1. Add 2 µl of Cloning Enhancer to 5 µl of the PCR reaction. Protocol BREAK 2. Incubate at 37°C for 15 minutes, then at 80°C for 15 minutes in a PCR thermal cycler. If you used more than 100 ng of DNA as a template in the PCR reaction, extend the 37°C incubation step to 20 minutes. If you are using a water bath or heat block rather than a thermal cycler, extend each of the incubation steps to 20–25 minutes. 3. Proceed with the In-Fusion Cloning Procedure for Cloning Enhancer-Treated PCR Inserts (Section VII.B). If you cannot proceed immediately, store treated PCR reactions at –20°C until you are ready. B. In-Fusion Cloning Procedure for Cloning Enhancer-Treated PCR Inserts Attention IMPORTANT: Before proceeding to the cloning reaction, be sure your target insert has been pretreated with the Cloning Enhancer, as described in Section A (above). DO NOT follow this procedure if your insert has been purified. 1. Use Table II to determine the amount of linearized vector to use in your In-Fusion reaction. Table II. Recommended amount of vector per in-fusion reaction Protocol Vector Size Recommended Nanograms (ng) <4 kb 100 ng 4 to 6 kb 100 to 150 ng 6 to 10 kb 200 ng >10 kb Up to 400 ng Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 12 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual VII. Protocol I: In-Fusion Cloning Procedure w/Cloning Enhancer, continued 2. Use Table III to determine the amount of Cloning Enhancer-Treated PCR Insert (from Section VII, Part A) to use in your In-Fusion reaction. Table III. Recommended AMOUNT of cloning enhancer-treated insert per in-fusion reaction* Protocol InsertSize Recommended Microliters (µl) <1 kb 1 µl 1 to 4 kb 1 to 2 µl 4 to 8 kb 4 µl 8 to 12 kb 7 µl *If you obtain a low product yield from your PCR reaction, we recommend adding more of the Cloning Enhancer treated insert (up to 7 µl). 3. Set up the In-Fusion cloning reaction: 5X In-Fusion Reaction Buffer 2 µl In-Fusion Enzyme Vector 1 µl x µl* Cloning Enhancer-Treated PCR Insert x µl* dH2O (as needed) Total Volume x µl 10 µl *For reactions with larger volumes of vector and insert (> 7 µl of vector + insert), double the amount of reaction buffer and enzyme, and add dH20 for a total volume of 20 µl. 4. Adjust the total reaction volume to 10 µl using deionized H2O and mix the reaction. 5. Incubate the reaction for 15 min at 37°C, followed by 15 min at 50°C, then place on ice. 6. Bring the reaction volume up to 50 µl* with TE buffer (pH 8), and mix well. *For some cell strains, it may be better to dilute the reaction with TE buffer to a volume of 100 μl. If your cloning efficiency is low, you may obtain better results if you dilute the reaction further. 7. BREAK Continue to the Transformation Procedure (Section IX). If you cannot transform cells immediately, store the cloning reactions at –20°C until you are ready. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 13 In-Fusion™ Advantage PCR Cloning Kit User Manual VIII. Protocol II: In-Fusion Cloning Procedure w/Spin-Column Purification A. Procedure for Spin-Column Purification of PCR Inserts Protocol 1. If non-specific background bands are observed on an agarose gel, isolate your target fragment by gel extraction, then spin-column purify. If a single band of the desired size is obtained, add 1 μl of DpnI to 50 μl of the PCR reaction and incubate at 37°C for 60 min, then spin-column purify. 2. Spin-column purify your PCR product (e.g., insert) by using a silica-based purification system, such as NucleoSpin® Extract II. During purification, avoid nuclease contamination and exposure of the DNA to UV light for long periods of time. 3. After purification, proceed with the In-Fusion Cloning Procedure for Spin Column-Purified PCR Inserts (Section VIII.B). B. In-Fusion Cloning Procedure for Spin-Column Purified PCR Inserts In general, maximum cloning efficiency is achieved when using a 2:1 molar ratio of insert:vector. Typically, 100 ng of a 4–5 kb linearized vector plus 50 ng of a 1 kb PCR product is found to work well in an In-Fusion reaction. If the size of your vector or PCR product is different from this, adjust the amount of your input DNA. Clontech provides an online tool to assist in determining the correct amount of insert and vector to achieve a 2:1 ratio (http://bioinfo.clontech.com/infusion/). Table Iv. Recommended in-fusion reactions for purified inserts Rxn Component Cloning Rxn Negative Control Rxn Positive Control Rxn Purified PCR insert 50–200 ng – 2 µl** Linearized vector 100–400 ng 1 µl* 1 µl* 5X In-Fusion Reaction Buffer 2 µl 2 µl 2 µl In-Fusion Enzyme 1 µl 1 µl 1 µl Deionized water to 10 µl to 10 µl to 10 µl *Use 1.0 µl of the linearized pUC19 Control Vector (50 ng/µl) included in the Kit. **Use 2 µl of the 2 kb Control Insert (40 ng/ml) included in the Kit. 1. Mix your purified PCR insert and vector together in a 2:1 molar ratio. 2. Set up the In-Fusion cloning reaction: Protocol 5X In-Fusion Reaction Buffer 2 µl In-Fusion Enzyme 1 µl Vector x µl* Purified PCR Insert x µl* dH2O (as needed) x µl Total Volume 10 µl *For reactions with larger volumes of vector and insert (> 7 µl of vector + insert), double the amount of reaction buffer and enzyme, and add dH20 for a total volume of 20 µl. 3. Adjust the total reaction volume to 10 µl using deionized H2O and mix the reaction 4. Incubate the reaction for 15 min at 37°C, followed by 15 min at 50°C, then place on ice. 5. Bring the reaction volume up to 50 µl* with TE buffer (pH 8) and mix well. *For some cell strains, it may be better to dilute the reaction to 100 μl with TE buffer If your cloning efficiency is low, you may obtain better results if you dilute the reaction further. BREAK 6. Continue to the Transformation Procedure (Section IX). If you cannot transform cells immediately, store the cloning reactions at –20°C until you are ready. Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 14 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual IX. Transformation Procedure Protocol In addition to the cloning reaction, we recommend that you perform positive and negative control transformations. The positive control should consist of a circular vector of known concentration (competent cells should give >1 x 108 cfu/µg), and the negative control should consist of a known amount of your linearized vector (see Section X for Expected Results). 1. Transform competent cells with 2.5 µl of the diluted In-Fusion reaction mixture. Follow the transformation protocol provided by your competent cell manufacturer. IMPORTANT: DO NOT add more than 5 µl of the diluted reaction to 50 µl of competent cells. More is not better. Using too much of the reaction mixture inhibits the transformation. For example, 0.5–1 µl of an undiluted In-Fusion reaction in 50 µl of cells typically yields over 1,000 colonies, while 2 µl of the same reaction will yield fewer than 100 colonies. Since it can be difficult to pipette 1 µl accurately, it is necessary to dilute the In-Fusion reaction with TE buffer (see Section VII.B.6 and Section VIII.B.5) before performing the transformation. Attention 2. Place 1/10th of each transformation reaction (25–50 µl) into separate tubes and bring the volume to 100 µl with SOC medium. Spread each diluted transformation reaction on a separate LB plate containing an antibiotic appropriate for the cloning vector (i.e., the control vector included with the Kit requires 100 µg/ml of ampicillin). 3. Centrifuge the remainder of each transformation reaction at 6000 rpm for 5 min. Discard the supernatant and resuspend each pellet in 100 µl fresh SOC medium. Spread each sample on a separate LB plate containing the appropriate antibiotic. Incubate all of the plates overnight at 37°C. 4. The next day, pick individual isolated colonies from each experimental plate. Isolate plasmid DNA using a standard method of your choice (e.g. miniprep). To determine the presence of insert, analyze the DNA by restriction digestion or PCR screening. X. Expected Results The positive control plates typically develop several hundred white colonies when using cells with a minimum transformation efficiency of 1 x 108 cfu/μg. The negative control plates should have few colonies. The number of colonies on your experimental plates will depend on the amount and purity of the PCR product and linearized vector used for the In-Fusion cloning reaction. • The presence of a low number of colonies on both plates—typically, a few dozen colonies— is indicative of either transformation with too much of the reaction, or poor DNA/primer quality. • The presence of many (hundreds) of colonies on the negative control is indicative of incomplete vector linearization. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 15 In-Fusion™ Advantage PCR Cloning Kit User Manual XI. Troubleshooting Guide If you do not obtain the expected results, use the following guide to troubleshoot your experiment. To confirm that your kit is working properly, perform the control reactions. Table V. Troubleshooting Guide for In-Fusion EXPERIMENTS A. No or Few Colonies Obtained from Transformation Description of Problem Low transformation efficiency Explanation Solution Transformed with too much In-Fusion reaction Do not add more than 5 µl of the diluted In-Fusion reaction to 50 µl of competent cells (see Section IX for details). Suboptimal dilution of In-Fusion reaction For some cell strains, it may be better to dilute the In-Fusion reaction with TE buffer up to a total volume of 100 μl (see Section VII.B, Step 6 OR Section VIII.B, Step 5). Suboptimal PCR product Repeat PCR amplification and purify product using a different method of purification. Alternatively, perform phenol:chloroform extraction on your original PCR product, followed by ethanol precipitation. Bacteria were not competent Check transformation efficiency. You should obtain >1 x 108 cfu/µg; otherwise use fresh competent cells. It is imperative to obtain the highest DNA concentration Low DNA concentration possible in your In-Fusion reaction. Either the amount of vector in reaction or the amount of PCR fragment was too low. We recommend using between 100 ng and 400 ng of vector, depending on its size (see Table II). Wrong molar ratio Low quality DNA fragments Gel purification introduced contaminants Primer sequences are incorrect Low cloning Cloning Enhancer may efficiency in the not have been experiment, but inactivated properly not in the control The molar ratio of PCR fragment to linear vector used in the In-Fusion protocol may not have been optimal. We recommend using between 100 ng and 400 ng of vector, and 50 to 200 ng of insert. Clontech provides an online tool to assist in determining the correct amount of insert and vector to achieve a 2:1 ratio (http://bioinfo.clontech.com/infusion/). If your insert was gel purified, it is imperative to obtain the highest DNA concentration possible in your In-Fusion reaction. The total volume of purified vector and insert should not exceed 5 µl. When possible, optimize your PCR amplification reactions such that you generate pure PCR products and use the Cloning Enhancer (see Section VII.A for details). Check primer sequences to ensure that they provide 15 bases of homology with the region flanking the insertion site (see Section V.B). Extend the inactivation step of the Cloning Enhancer to 80°C for 20–25 minutes. Protocol No. PT4065-1 www.clontech.com Version No. PR9Z3431 16 Clontech Laboratories, Inc. A Takara Bio Company In-Fusion™ Advantage PCR Cloning Kit User Manual XI. Troubleshooting Guide, continued Table V. Troubleshooting Guide for In-Fusion EXPERIMENTS B. Large Numbers of Colonies Contained No Insert Description of Problem Explanation Solution Incomplete linearization It is important to remove any uncut vector prior to use in the In-Fusion reaction. If necessary, recut your of your vector vector and gel purify. Large numbers of Contamination of colonies obtained with In-Fusion reaction by no insert plasmid with same antibiotic resistance Plates too old or contained incorrect antibiotic If your insert was amplified from a plasmid, closed circular DNA may have carried through purification and contaminated the cloning reaction: a) To ensure the removal of any plasmid contamination, we recommend linearizing the template DNA before performing PCR. b) If you spin-column purify your insert, be sure to treat the PCR product with DpnI before purification in order to remove contaminating template DNA (see Section VIII.A.1). Be sure that your antibiotic plates are fresh (<1 month old). Check the antibiotic resistance of your fragment. C. Clones Contained Incorrect Insert Large number of colonies contain incorrect insert Your PCR product contained non-specific sequences If your PCR product is not a single distinct band, then it may be necessary to gel purify the PCR product to ensure cloning of the correct insert. See Section VI for more information. Notice to Purchaser Clontech products are to be used for research purposes only.They may not be used for any other purpose, including, but not limited to, use in drugs, in vitro diagnostic purposes, therapeutics, or in humans. Clontech products may not be transferred to third parties, resold, modified for resale, or used to manufacture commercial products or to provide a service to third parties without written approval of Clontech Laboratories, Inc. This product is covered by U.S. Patent No 7,575,860 and European Patent No. EP1741787. Fusion-Blue™ Cells are manufactured and tested by Novagen for Clontech Laboratories, Inc. Novagen® is a registered trademark of EMD Biosciences, Inc. U.S. Patent No. 5,436,149 for LA Technology is owned by Takara Bio Inc. TaqStart® Antibody and other Hot Start Antibodies are licensed under U.S. Patent No. 5,338,671. DH5™, DH5a™, DH10B™ and ElectroMax™are trademarks, and Max Efficiency® is a registered trademark of Invitrogen Corporation. NucleoSpin® and NucleoTrap® are registered trademarks of MACHEREY-NAGEL GmbH and Co., KG. Clontech, the Clontech Logo and all other trademarks are the property of Clontech Laboratories, Inc., unless noted otherwise. Clontech is a Takara Bio Company. ©2010 Clontech Laboratories, Inc. Clontech Laboratories, Inc. www.clontech.com A Takara Bio Company Protocol No. PT4065-1 Version No. PR9Z3431 17