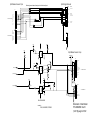
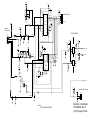
Download GE Dinamap ProCare Service manual
Transcript
DINAMAP® ProCare Monitor Service Manual This manual is for DINAMAP ProCare Monitors models 100, 200, 300, and 400, with or without printers. • ProCare 100: BP, Pulse • ProCare 200: BP, Pulse, and Temp • ProCare 300: BP, Pulse, and SpO2 • ProCare 400: BP, Pulse, Temp, and SpO2 The model of the Monitor determines which parameters are in your monitor. Please refer to applicable sections. Reissues and Updates Changes occurring between issues are addressed through Change Information Sheets, Addendums, and replacement pages. If a Change Information Sheet does not accompany this manual, it is correct as printed. Errors and Omissions If errors or omissions are found in this manual, please notify: GE Medical Systems Information Technologies Technical Publications 4502 Woodland Corporate Boulevard Tampa, FL 33614 1-877-274-8456 Part No. 2009381-001 B The content of this document including all figures and drawings is proprietary information of GE Medical Systems Information Technologies, provided solely for purposes of operation, maintenance or repair, and dissemination for other purposes or copying thereof is prohibited without prior written consent by GE Medical Systems Information Technologies, Tampa, Florida. Illustrations may show design models; production units may incorporate changes. Hierarchy of Warnings and Cautions A general warning is a statement that alerts the user to the possibility of injury, death, or other serious adverse reactions associated with the misuse of the device. A warning relates to steps in a procedure. A general caution is a statement that alerts the user to the possibility of a problem with the device associated with its use or misuse. Such problems include device malfunction, device failure, damage to the device or damage to other property. A caution relates to steps in a procedure. © GE Medical Systems Information Technologies 2002, 2004 TAMPA, FL. All rights reserved. Revision B ProCare Patient Monitor Service Manual 2009381-001 World Headquarters GE Medical Systems Information Technologies, Inc. 8200 West Tower Avenue Milwaukee, WI 53223 USA Tel:•+ 1 414 355 5000 •1 800 558 5120 (US only) Fax:•+ 1 414 355 3790 European Representative GE Medical Systems Information Technologies GmbH Munzinger Straße 3-5 D-79111 Freiburg Germany Tel: •+ 49 761 45 43 - 0 Fax: •+ 49 761 45 43 - 233 Asia Headquarters GE Medical Systems Information Technologies Asia; GE (China) Co., Ltd. 24th Floor, Shanghai MAXDO Center, 8 Xing Yi Road, Hong Qiao Development Zone Shanghai 200336, P.R. China Tel: •+ 86 21 5257 4650 Fax: •+ 86 21 5208 2008 ProCare Patient Monitor Service Manual 2009381-001 Revision B DINAMAP® ProCare Monitor Service Manual 2009381-001 Revision B NOTE: The information in this manual only applies to ProCare Monitor. It does not apply to earlier Monitors. Due to continuing product innovation, specifications in this manual are subject to change without notice. © GE Medical Systems Information Technologies, 2002, 2004. All rights reserved. T-2 ProCare Monitor 2009381-001 Revision B Table of Contents 1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .. . . . . 1-1 1.1 Scope of Manual . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3 1.2 Manual Changes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-4 1.3 Service Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5 1.3.1 Service Contracts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5 1.3.2 Assistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-5 1.3.3 Return to Factory Repair Service . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1- 5 1.3.4 Service Loaners . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6 1.3.5 Repair Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-6 1.3.6 Replacement Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-7 1.4 Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-8 1.4.1 General Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1- 8 2 Product Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1 2.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 2.2. Product Configurations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3 2.3. Controls, Indicators, and Connectors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4 2.3.1. ProCare Monitor Rear Panel Connections . . . . . . . . . . . . . . . . . . . . . . . 2-4 2.3.2. Front Panel Controls and Indicators . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-5 2.4. Host Communications Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9 2.4.1. DB15 Connector Pin Assignments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-9 2.5. Compatible Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-10 2.6. Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-12 3 Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1 3.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3 3.2 Overall Principles of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4 3.2.1 SpO2 (Model 300 and 400) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4 Revsion B ProCare Patient Monitor Service Manual 2009381-001 Table of Contents 3.2.2 Cuff Blood Pressure (BP) and Pulse . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-4 3.2.3 Temperature (Model 200 and 400) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5 3.2.4 Host Communication Port . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5 3.3 Functional Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-5 3.3.1 Main Board PWA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6 3.3.2 User Interface (UI) Board PWA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6 3.3.3 SPO2 PWA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7 3.3.4 Printer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7 3.3.5 Pneumatic Valve/Manifold (PVM) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7 3.3.6 Optical Switch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7 4 Calibration & Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . 4-1 4.1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4- 3 4.2 Configuring Your ProCare Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4 4.2.1 Unpacking and Preparation for Installation . . . . . . . . . . . . . . . . . . . . . . . 4-4 4.2.2 Set the Date and the Clock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-4 4.2.3 Parameter Level Functional Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5 4.3 Periodic Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6 4.3.1 As Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-6 4.3.2 Annual Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-7 4.4 Care of the Storage Battery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8 4.4.1 Battery Charging . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-8 4.5 Safety Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9 Notes on Electrical Safety Testing of the ProCare Monitor: . . . . . . . 4-9 4.5.1 Temp Circuit Leakage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10 4.5.2 SpO2 Circuit Leakage Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10 4.6 ProCare Patient Monitor Parameter Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11 4.6.1 SETUP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-11 4.6.2 Leakage Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12 4.6.3 Pressure Transducer Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-12 4.6.4 Pressure Transducer Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-13 4.6.5 Overpressure Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14 4.6.6 Button Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-14 4.6.7 LED Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15 4.6.8 External DC Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15 4.6.9 NIBP Determination . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-15 4.6.10 NIBP Overpressure Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16 ProCare Patient Monitor Service Manual 2009381-001 Revision B Table of Contents 4.6.11 Temperature (Perform if equipped with Temp module) . . . . . . . . . . . 4.6.12 SpO2 (Perform only if equipped with SpO2 module) . . . . . . . . . . . . . 4.6.13 Printer Output Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4.6.14 Communication Port Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16 4-17 4-18 4-18 4.7 Alarm Code Interpretation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19 4.7.1 System Failures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19 Appendix A - Test Results Form . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4- 21 Appendix B - Connectivity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4- 23 Appendix C - Display Cover: Removal, Installation . . . . . . . . . . . . . . . . . . . . . . . . . 4-25 Appendix D: Replacement Parts and Assemblies . . . . .. . . . . . . . . . . . . . . . . . . . . . 4-27 Appendix E: Electromagnetic Compatibility . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-37 5 Revsion B Assembly Drawings & ProCare Schematics. . . . . . . . . . . . . . . . . . 5-1 ProCare Patient Monitor Service Manual 2009381-001 Table of Contents ProCare Patient Monitor Service Manual 2009381-001 Revision B 1 Revision B Introduction ProCare Patient Monitor Service Manual 2009381-001 1-1 1-2 ProCare Patient Monitor Service Manual 2009381-001 Revision B 1.1 Scope of Manual This Service Manual provides service and parts repair information about the DINAMAP ProCare Monitor. This manual is intended for use by trained service technicians who are familiar with electromechanical devices and analog circuit techniques. WARNING To reduce the risk of electric shock, do not remove cover or back of any component. Refer servicing to qualified service personnel. Only qualified technicians should perform repairs to this equipment. For information about operating the Monitor in a clinical environment, refer to the separate Operations Manual. This Service Manual consists of the following five sections: Section 1 describes this volume and tells you how to use it. Information is also provided about the physical and functional characteristics of the Monitor, and how to get assistance in the event the unit fails to function properly. Section 2 provides a general overview of the ProCare Monitor including the user controls, external connections and product/parameter specification. Section 3 presents principles of operation for the Monitor, including an overall system description and principles of operation at the component level. Section 4 provides information about periodic and corrective maintenance of the Monitor and part list, replacement part lists. Procedures include module performance testing, and calibration procedures. Information is also provided to facilitate isolating faults to the subassembly level. Section 5 provides component information about the Monitor, including assembly drawings and electrical schematics. 1-3 ProCare Patient Monitor Service Manual 2009381-001 Revision B Introduction: 1.2 Manual Changes 1.2 Manual Changes If, in the normal course of using this manual, you notice errors, omissions, incorrect data, or if you can suggest comments that may help improve this manual, please send suggestion to: General Electric Medical Systems Information Technologies Technical Publications 4502 Woodland Corporate Boulevard Tampa, Florida, 33614 Changes to the Service Manual, either in response to user input or to reflect continuing product improvements, are accomplished through reissue. Changes occurring between reissues are addressed through Change Information Sheets and replacement pages. If a Change Information Sheet does not accompany your manual, the manual is correct as printed. 1-4 ProCare Patient Monitor Service Manual 2009381-001 Revision B Introduction: 1.3 Service Policy 1.3 Service Policy The warranty for this product is two years Parts and Labor. In the event of a monitor malfunction the monitor must be returned to General Electric Medical Systems Information Technologies to be covered under warranty. All repairs on products under warranty must be performed or approved by Product Service personnel. Unauthorized repairs will void the warranty. Only qualified electronics service personnel should repair products not covered by warranty. 1.3.1 Service Contracts Service contracts may be purchased on most products. Contact your Sales Representative for details and pricing. 1.3.2 Assistance If the product fails to function properly, or if assistance, service or spare parts are required, contact Customer Support. Before contacting Customer Support, it is helpful to attempt to duplicate the problem and to check all accessories to ensure that they are not the cause of the problem. If you are unable to resolve the problem after checking these items, contact General Electric Medical Systems Information Technologies at 1-877-274-8456. Prior to calling, please be prepared to provide: product name and model number a complete description of the problem If repair parts or service are necessary, you will also be asked to provide: the product serial number the facility's complete name and address a purchase order number if the product is in need of non-warranty repair or to order spare parts the facility's General Electric Medical Systems Information Technologies account number the appropriate part number for spare or replacement parts 1.3.3 Return to Factory Repair Service If your product requires warranty, extended warranty or non-warranty repair service, call Customer Support and a representative will assist you. Estimates for non-warranty repairs are provided pre-set flat rates to facilitate prompt service. In cases where the product has external chassis or case damage, please advise the Customer Support representative when you call. The Customer Support representative will record all necessary information and will provide you with a Return Merchandise Authorization Number (RMA). Prior to returning any product for repair, you must have a RMA number. Revsion B ProCare Patient Monitor Service Manual 2009381-001 1-5 Introduction: 1.3 Service Policy Contact General Electric Medical Systems Information Technologies at 1-877-274-8456 Monday through Friday, 8:00 a.m. to 6:00 p.m. EST, excluding holidays. Packing Instructions Follow these recommended packing instructions. Remove all hoses, cables, sensors, and power cords from the monitor before packing. Pack only the accessories you are requested to return; place them in a separate bag and insert the bag and the product inside the shipping carton. Use the original shipping carton and packing materials, if available. If the original shipping carton is not available: Place the product in a plastic bag and tie or tape the bag to prevent loose particles or materials from entering openings such as hose ports. Use a sturdy corrugated container to ship the product. Pack with 4 to 6 in. of padding on all sides of the product. Tape securely to seal the container for shipping. Insurance Insurance is at the customer's discretion. The shipper must initiate claims for damage to the product incurred during shipping. Shipping damage is not covered under warranty. 1.3.4 Service Loaners Loaner units can be provided at no charge during the warranty period if your product needs to be returned for factory service. If requested, loaner units will be shipped within 48 hours to your facility. General Electric Medical Systems Information Technologies will pay outgoing charges for a loaner sent for product repairs under warranty. The customer is responsible for shipping charges to return the loaner unit to General Electric Medical Systems Information Technologies. All loaner units must be returned within 5 business days after receipt of your repaired unit. Loaner and/or Rental products are available to meet your needs in nonwarranty situations. 1.3.5 Repair Parts Repair parts can be ordered from General Electric Medical Systems Information Technologies: Via phone: 1-877-274-8456, or Via FAX: 1-800-421-6841 Exchange replacement assemblies such as Circuit Board Assemblies are also available; ask your Customer Support representative for details. 1-6 ProCare Patient Monitor Service Manual 2009381-001 Revision B Introduction: 1.3 Service Policy Most orders ship on the same day if received before 3PM Eastern Time. Overnight freight options are available to meet your critical needs. All orders must include the following information: Facility's complete name, address, and phone number FAX number Your purchase order number Your General Electric Medical Systems Information Technologies account number (if available) 1.3.6 Replacement Accessories Replacements such as hoses, sensors, etc. must be purchased from General Electric Medical Systems Information Technologies at 1-800-5585102. Please have the Product Code of the item you wish to order, your purchase order and account number available. Revsion B ProCare Patient Monitor Service Manual 2009381-001 1-7 Introduction: 1.4 Product Description 1.4 Product Description The Monitor is described below. Refer to Table 1-1 for specifications. 1.4.1 General Description The ProCare Monitor provides a small, portable, easy-to-use monitoring alternative for sub-acute hospital and non-hospital settings. The DCoperated Monitor offers noninvasive determination of systolic blood pressure, diastolic blood pressure, mean arterial pressure, pulse rate, oxygen saturation, and temperature. Monitors are available with or without integrated printers. ProCare Monitors are intended for use in various markets, from the physician’s office to sub-acute triage and medical/surgical units. Indicators for external DC operation (from AC mains), battery operation, and battery charging are at the front of the unit. At the time of publication, the available functioning parameters included the following: NIBP Nellcor™ Pulse oximetry (SpO2) Masimo Pulse oximetry (SpO2) Alaris™ Oral and Rectal thermometry thermal recorder/printer Other DINAMAP ProCare features include: The ability to uses industry standard accessories Remote alarm capability Function keys for quick access to Alarm Silence, Monitor Menus, Print and NIBP Inflate/Stop The ProCare Monitor operates from either an external DC power supply, or from the internal lead-acid storage battery. When external DC power becomes available, the system rapidly switches from battery power to external power. 1-8 ProCare Patient Monitor Service Manual 2009381-001 Revision B 2 Revsion B Product Description ProCare Partient Monitor Service Manual 2009381-001 2-1 For your notes 2-2 ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.1 Introduction 2.1 Introduction DINAMAP ProCare Monitors provide non-invasive determination of systolic blood pressure, diastolic blood pressure, mean arterial pressure, pulse rate, temperature, and oxygen saturation. 2.2. Product Configurations Each ProCare Monitor is supplied with an accessory pack. The contents of the pack vary according to model. Unpack the items carefully, and check them against the checklists enclosed within the accessory boxes. If an accessory is missing or if an item is in a nonworking condition, contact General Electric Medical Systems Information Technologies Customer Service immediately. It is recommended that all the packaging be retained, in case the ProCare Monitor must be returned for service in the future. Revision B ProCare Partient Monitor Service Manual 2009381-001 2-3 Product Description: 2.3. Controls, Indicators, and Connectors 2.3. Controls, Indicators, and Connectors Descriptions of the items shown are listed on the pages that follow. For symbol definitions, refer to page 2-8 of this section. 2.3.1. ProCare Monitor Rear Panel Connections 1 Speaker Grille. 2 Data interface connector: Host communications port (15 pin D-type RS232 serial port) for use only with equipment conforming to IEC 601-1, configured to comply with IEC 601-1. 3 Printer Door (optional.) 2-4 ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.3. Controls, Indicators, and Connectors 2.3.2. Front Panel Controls and Indicators Buttons 1 Silence button: Press to mute audible alarms. Any alarm active that is acknowledgeable is also removed whenever this key is pressed. When pressed after alarm sounds (silence active), the silence icon (bell) lights to indicate that audible alarms have been silenced for 2 minutes. 2 Alarms button: Press to view or adjust parameter alarm settings 3 +/- button (Plus/Minus): Press the + button to increment an adjustable setting and the - button to decrement an adjustable setting. This button is active only when a user-setting mode (limit or menu) is active. 4 Menu button: Press to access menu settings that can be adjusted while in clinical mode (i.e., ALARM VOLUME, PULSE VOLUME, INFLATE PRESSURE; refer to Operating Modes in this section for a description of clinical mode.) 5 SpO2 sensor connector: SpO2 sensor cable attaches here . 6 BP connector: BP cuff hose attaches here. 7 Inflate/Stop button: Press to start a manual BP determination or stop any BP determination. 8 Temperature probe holster: Temperature probe is stored here. 9 Cycle button: Press to start Auto Cycle or STAT mode. 10 Temperature probe cover storage: Box of probe covers is stored here 11 History button: Press to activate the history mode. When activated, it displays the most recent entries stored. Press and hold the button for 2 seconds to clear all entries stored. 12 Print button: Press to print currently displayed values or all stored entries when in history mode. 13 On/Off button: Controls on/off state of monitor; push for power on and push again for power off. 14 Temperature probe connector: Temperature probe cable attaches here. Revision B ProCare Partient Monitor Service Manual 2009381-001 2-5 Product Description: 2.3. Controls, Indicators, and Connectors Front Panel 15 Silence icon: when Silence button is pressed after alarm sounds (silence active), silence icon (bell) lights to indicate that audible alarms have been silenced for 2 minutes. 16 Systolic window: 3-digit red LED indicates measured systolic Blood Pressure in mmHg. 17 Diastolic window: 3-digit red LED indicates measured diastolic Blood Pressure in mmHg. 18 Alarm volume indicator: lights to indicate you are making a change to the alarm volume. 19 Pulse volume: illuminates to indicate you are making a change to the pulse volume. 20 Inflate pressure: illuminates to indicate you are making a change to the inflation pressure. 21 Pulse Rate window: 3-digit yellow LED shows the pulse rate in beats per minute. 22 SpO2 pulse indicator: Red LED bar flashes to indicate that real-time pulse rate measurements are being derived from SpO2 signals. 23 SpO2 window: 3-digit red LED indicates oxygen saturation in %. 24 MAP/Cuff window: 3-digit red LED indicates measured MAP in mmHg and shows instantaneous cuff pressure during BP determination. 25 Min window: Displays the BP mode if manual or STAT is the cycle time when in Auto Cycle mode. 26 Battery power indicator: Green LED indicates the Monitor is operating on battery power. 27 Low battery power indicator: Yellow LED indicates LOW charge status of internal battery. 28 Charging indicator: Green LED indicates presence of external power source and battery charging. 29 Temperature window: 4-digit red LED indicates measured temperature. 2-6 ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.3. Controls, Indicators, and Connectors Right-Side Panel 30 External power socket: To be used with approved GE Medical Systems Information Technologies AC-DC power converter ONLY. Revision B ProCare Partient Monitor Service Manual 2009381-001 2-7 Product Description: 2.3. Controls, Indicators, and Connectors Symbols The following symbols are associated with the ProCare Monitor. Note: The model of the Monitor determines which symbols appear on it. Attention, consult accompanying documents Silence Alarms +/Menu Inflate/Stop Cycle History Print On/Off Battery Power External Communications Port Connector Charging Defibrillator-proof type BF equipment Class II equipment according to IEC 60536 Packaging label depicting the transportation and storage atmospheric pressure range of 500 to 1060 hPa. 2-8 ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.4. Host Communications Connector 2.4. Host Communications Connector All host port signals are NON-ISOLATED and should be connected to equipment conforming to IEC 601-1 ONLY. Where isolation of data communication is required, the isolated level converter should be used. If external alarm control is required, p/n 487208 (Isolated Remote Alarm Cable Assembly) should ALWAYS be used. Please refer to the Information Sheet included with the isolated remote alarm cable for operational details. Note: When using remote alarm, the ProCare Monitor should be considered the primary alarm source. The secondary alarm is used for secondary purposes only. 2.4.1. DB15 Connector Pin Assignments Connection Details Host Port Connector (rear panel) WARNING! Auxiliary equipment connected to the DINAMAP® ProCare Monitor will result in the formation of an electromedical system and thus must comply with the requirements of EN 60601-1-1/ IEC 601-1. All host port signals are NON-ISOLATED and should be connected to equipment conforming to IEC-601-1, configured to comply with IEC 601-1-1 ONLY. Where isolation of data communication is required, GE Medical Systems Information Technologies part number ILC1926 should be used. If external alarm control is required, GE Medical Systems Information Technologies part number 487208 (Isolated Remote Alarm Cable Assembly) should ALWAYS be used. When a high-priority condition is displayed on the Monitor, the remote alarm signal becomes active within 0.5 seconds. The active state of the alarm signal is an open circuit. In the inactive state the alarm signal is connected to ground. Please refer to the Information Sheet included with the isolated remote alarm cable for operational details. Note: When using remote alarm, the ProCare Monitor should be considered the primary alarm source. The secondary alarm is used for secondary purposes only. Pin # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Revision B Function Common Inverted TTL Transmit Data Inverted TTL Receive Data +5 volts No connection No connection Common Remote Alarm No connection No connection RS232 Transmit Data (TxD) No connection RS232 Receive Data (RxD) No connection No connection ProCare Partient Monitor Service Manual 2009381-001 2-9 Product Description: 2.5. Compatible Parts 2.5. Compatible Parts Reorder Codes Product Code ECAT DINAMAP ProCare Monitor Operations Manual 2009360-001 DINAMAP ProCare Monitor Service Manual 2009381-001 Battery 633178CR Power Cord 316579 Printer Paper (box of 10) 089100 E9050KP DINAMAP Rolling Stand 003215 E9050JB Pole Mounting Option 2009762-001 Wall Mounting Option 2009763-001 Hose Management Option 2009764-001 Bed Rail Option 2009765-001 Nurse Call 487208CR NIBP OPTIONS: Airhose 12 ft Adult/Pediatric, Screw Connector 107365 E9050LH Airhose 24 ft Adult/Pediatric, Screw Connector 107366 E9050LJ Airhose 12 ft Neonatal, Quick Disconnect 107368 E9050LK Airhose 12 ft Adult/Pediatric, Quick Disconnect 88847 E9050KN CLASSIC-CUF® Assortment pack Includes 1 each: Infant, Child, Small Adult, Adult, Large Adult, Thigh Cuff 2692 E2692J CLASSIC-CUF® Assortment Pack, Neonate Includes 2 Neo #1, 3 Neo #2, 5 Neo #3, 5 Neo #4, 5 Neo #3 2693 E2693J SOFT-CUF® Assortment Pack Includes 1 each: Infant, Child, Small Adult, Adult, Large Adult, Thigh Cuff 2695 E2695J SOFT-CUF® Assortment, Neonate Includes 2 Neo #1, 3 Neo #2, 5 neo #3, 5 Neo #4, 5 Neo #5 2694 E2694J DURA-CUF® Assortment Pack Includes 1 each: Infant, Child, Small Adult, Adult, Large Adult, Thigh Cuff 2699 E2699J DURA-CUF® Assortment Pack, Adult Includes 1 each: Infant, CHild, Small Adult, Adult, Large Adult, Thigh Cuff 2698 E2698J CUFF ASSORTMENT PACKS: 2-10 ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.5. Compatible Parts Reorder Codes Product Code DURA-CUF® Assortment Pack, Child Includes 2 Infant, 3 Child, and 1 Small Adult Cuff 2697 ECAT E2697J Additional Blood Pressure Cuffs are available through http://www.gemedicalsystems.com TEMPERATURE IVAC* Turbo Temp Oral Temperature Probe, Long Cord 2008774-001 IVAC* Turbo Temp Rectal Temperature Probe, Long Cord 2008775-001 IVAC* Temperature Probe Covers 88015 E9050KK SpO2 Nellcor**: Pulse Oximeter Cable DOC-10 2008773-001 DuraSensor Adult Oxygen Sensor DS100A Masimo***: Adult Reusable Sensor, 1/Bx (NR125) 2009745-001 Cable (PC08) 2009743-001 * IVAC is a trademark of Alaris Medical Systems ** NELLCOR is a trademark of Mallinckrodt *** Masimo is a trademark of Masimo Corporation Revision B ProCare Partient Monitor Service Manual 2009381-001 2-11 Product Description: 2.6. Specifications 2.6. Specifications 0 0 8 6 IPX1 This product conforms with the essential requirements of the Medical Device Directive. Accessories without the CE Mark are not guaranteed to meet the Essential requirements of the Medical Device Directive. The ProCare Monitor is protected against vertically falling drops of water and conforms to the IEC-529 standard at level IPX1. Vertically falling drops shall have no harmful effects to the monitor. Power Requirements MAINS AC INPUT VOLTAGE Protection against electrical shock - Class II 120 VAC/60 Hz 24W Alternate Sources Protection against electrical shock - Class II DC INPUT VOLTAGE 24 VDC (nominal), at 1A The AC Mains adapter contains a nonresettable and nonreplaceable fuse. Battery 6 volt, 3.3 amp-hours. Protected by internal auto-resetting fuse and thermal protection. Minimum Operation Time 2 hrs (5 min cycle with adult cuff at 25° C, SpO2 active at 60 bpm, temp in monitor mode, printout of current values every 5 minutes) from full charge. Fuses The Monitor contains four fuses. The fuses are auto-resettable and mounted within the Monitor. The fuses protect the low voltage DC input, the battery, the remote alarm output and the +5V output on the host port connector. Environmental Operating Temperature +5° C to + 40° C (-41 F to +104 F) Storage Temperature -20° C to +50° C (-4 F to +122 F) Operating Atmospheric Pressure Range 700 to 1060 hecto Pascal Humidity Range 0% to 95% non-condensing Radio Frequency Complies with IEC Publication 601-1-2 (April 1993) Medical Electrical Equipment, Electromagnetic Compatibility Requirements and Tests, and CISPR 11 (Group1, Class A) for radiated and conducted emissions. Mechanical Dimensions 2-12 Height: 9.8 in (25.0 cm) Width: 9.8 in (24.8 cm) Depth: 6.9 in (17.5 cm) Weight including battery 7.8 lb (3.5 Kg) Mountings Self Supporting on rubber feet. Portability Carried by built-in handle ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.6. Specifications Mechanical Classification Information Mode of Operation: Continuous degree of Protection against harmful ingress of water: Drip-Proof IPX1. NIBP CUFF PRESSURE RANGE Adult Neonate 0 mmHg to 290 mmHg 0 mmHg to 145 mmHg DEFAULT TARGET: CUFF INFLATION Adult Neonate 150 ± 15 mmHg 110 ± 15 mmHg TARGET CUFF INFLATION ADJUSTMENT RANGE Adult Neonate 100 - 250 mmHg (5mmHg Steps) 100 - 140 mmHg (5 mmHg Steps) BLOOD PRESSURE DETERMINATION TIME Adult Neonate 120 seconds maximum 85 seconds maximum PULSE RATE: RANGE Adult Neonate 30 - 200 BPM ±3% 30 - 220 BPM ±3% BLOOD PRESSURE: MEASUREMENT RANGES Adult Neonate NIBP ACCURACY Systolic mmHg MAP mmHg Diastolic mmHg 30 - 290 30 - 140 20 - 260 20 - 125 10 - 220 10 - 110 Meets AAMI/ANSI standard SP-10 AAMI/ANSI standard: ±5 mmHg mean error Intra-arterial method: ± 8 mmHg standard dev. Temperature - Alaris Thermometry SCALES Fahrenheit RANGE Max Min Celsius 42.2° Celsius 108.0° Fahrenheit 31.6° Celsius 88.9° Fahrenheit Monitor Mode Accuracy ±0.1° C ± 0.2° F (when tested in a calibrated liquid bath; meets ASTM E1112, Table 1 in range specified) Predictive Mode Accuracy ±0.6° C ±1.0° F Determination Time Less then 60 seconds SpO2 - Nellcor Pulse Oximetry Revision B SpO2 Range and Accuracy adult/neonate: 70-100% ±3.5 digits Pulse Rate Range and Accuracy 30 - 250 BPM ±3 BPM Saturation Pitch Indicator Pitch changes with saturation Sensor Connect/Disconnect from Patient The Monitor detects the connection or disconnection or a sensor to the patient within 15 seconds ProCare Partient Monitor Service Manual 2009381-001 2-13 Product Description: 2.6. Specifications SpO2 - Nellcor Pulse Oximetry Sensor Connect/Disconnect from Monitor The monitor detects the attachment or disconnection of a sensor from the Monitor within 5 seconds. Pulse Detection The Monitor detects a pulse of enters a no-signal state within 15 seconds of being attached to a patient. Loss of Pulse the monitor detects loss of pulse from patient and enters a no signal state within 10 seconds. Nellcor Sensors - SpO2 Range 70% to 100% OxiMAX Sensor Models Single Patient Use MAX-A,* MAX-AL* ±2 MAX-N†* ±2 MAX-P* ±2 MAX-I* ±2 MAX-R‡ ±3.5 OxiCliq Sensor Models Single Patient Use OxiCliq A ±2.5 OxiCliq P ±2.5 OxiCliq N† ±2.5 Reusable Sensor Models D-YS ±3 D-YS & D-YSE ±3.5 D-YS & DYSPD ±3.5 DS-100A ±3 OXI-A/N ±3 OXI-P/I ±3 * The accuracy specification under motion conditions is ±3. For a definition of motion contact GE Medical Services Technical Support. † The MAX-N and the OxiCliq N were tested on patients > 40 kg. ‡ The accuracy specification has been determined between saturations of 80-100% SpO2 - Masimo Oximetry 2-14 Saturation Range 1% - 100% Pulse Rate and Accuracy 25-240 BPM ±3 digits Perfusion Range 0.02 to 20% ProCare Partient Monitor Service Manual 2009381-001 Revision B Product Description: 2.6. Specifications SpO2 - Masimo Oximetry Accuracy and Motion Tolerance Without Motion - Adult/Ped* 70 to 100% ±2 digits Without Motion - Neonate* 70 to 100% ±3 digits With Motion - Adult/Ped/Neo**† 70 to 100% ±3 digits Low Perfusion‡ 70 to 100% ±2 digits 0 to 69% unspecified Pulse Rate Without Motion 25 to 240 beats/min ±3 digits With Motion normal physiologic range 25 to 240 beats/min ±5 digits * The Masimo SET® SpO2 parameter with LNOP-Adt sensors has been validated for no motion accuracy in human blood studies on healthy adult volunteers in induced hypoxia studies in the range of 70-100% SpO2 against a laboratory co-oximeter and ECG monitor. This variation equals plus or minus one standard deviation. Plus or minus one standard deviation encompasses 68% of the population. **The Masimo SET® SpO2 parameter with LNOP-Adt sensors has been validated for motion accuracy in human blood studies on healthy adult volunteers in induced hypoxia studies while performing rubbing and tapping motions at 2 to 4 Hz at an amplitude of 1 to 2 cm and a nonrepetitive motion between 1 to 5 Hz at an amplitude of 2 to 3 cm in induced hypoxia studies in the range of 70-100% SpO2 against a laboratory co-oximeter and ECG monitor. This variation equals plus or minus one standard deviation. Plus or minus one standard deviation encompasses 68% of the population. †The Masimo SET® SpO2 parameter with LNOP-Neo Pt sensors has been validated for neonatal motion accuracy in human blood studies on neonates while moving the neonate’s foot at 2 to 4 cm against a laboratory cooximeter and ECG monitor. This variation equals plus or minus, one standard deviation.Plus or minus one standard deviation encompasses 68% of the population. ‡The Masimo SET® SpO2 parameter with has been validated for low perfusion accuracy in bench top testing against a Biotek Index 2 stimulator and Masimo’s simulator with signal strengths of greater than 0.02% and a % transmission of greater than 5% for saturations ranging from 70 to 100%. This variation equals plus or minus, one standard deviation.Plus or minus one standard deviation encompasses 68% of the population. Revision B ProCare Partient Monitor Service Manual 2009381-001 2-15 Product Description: 2.6. Specifications 2-16 ProCare Partient Monitor Service Manual 2009381-001 Revision B 3 Revision B Principles of Operation ProCare Patient Monitor Service Manual 2009381-001 3-1 For your notes 3-2 ProCare Patient Monitor Service Manual 2009381-001 Revision B Principles of Operation: 3.1 Introduction 3.1 Introduction This section provides overall theory of operation and functional description of the ProCare Monitor. The ProCare Monitor comes in six different configurations: • ProCare 100 - Capable of monitoring Blood Pressure (BP) and Pulse • ProCare 200 - Capable of monitoring Blood Pressure (BP), Pulse, and Temperature • ProCare 300 Nellcor - Capable of monitoring Blood Pressure (BP), Pulse and SPO2 (Nellcor technology) • ProCare 300 Masimo - Capable of monitoring Blood Pressure (BP), Pulse and SPO2 (Masimo technology) • ProCare 400 Nellcor - Capable of monitoring Blood Pressure (BP), Pulse, SPO2 (Nellcor technology), and Temperature • ProCare 400 Masimo - Capable of monitoring Blood Pressure (BP), Pulse, SPO2 (Masimo technology), and Temperature The model of your monitor determines which parameters are in your monitor. Using the ProCare monitor, a clinician can view, print and recall data that is derived from each parameter. The Monitor is also capable of alerting the clinician to changes in the patient’s condition. All of the main operations of the ProCare Monitor are easy-to-use and only a button-touch away. Please review the factory default settings and, where applicable, enter settings appropriate for your use. Revsion B ProCare Partient Monitor Service Manual 2009381-001 3-3 Principles of Operation: 3.2 Overall Principles of Operation 3.2 Overall Principles of Operation The following paragraphs provide a general system interface relationship. The general block diagram is located in Figure 3-1. The ProCare Monitor is a portable unit that receives input power from an internal rechargeable battery. When the ON/OFF button is pressed, the Main Board is brought out of a sleep mode and turns on the power regulators. The power regulators provide conditioned power from the Lead Acid Battery. The external DC source is used only to charge the Lead Acid Battery. Once the ProCare Monitor is energized, a self-test is performed. The self-test automatically tests the main functions of the ProCare Monitor. Failure of the self-test will set the ProCare Monitor into a fail-safe mode with an audio alarm. Under normal operating condition, the ProCare Monitor is ready to record the patient vital signs using three external attachments: the temperature probe, SPO2 sensor, and cuff. Interface with a central station or other device is accomplished through the host communication port on the back of the ProCare Monitor. 3.2.1 SpO2 (Model 300 and 400) The SpO2 probe has a built-in sensor. When the SpO2 sensor is attached to the SpO2 connector and patient, the probe senses the heart rate and oxygen saturation. The analog signals are routed to the SpO2 PWA (Nellcor or Masimo). The analog signals are analyzed on the SpO2 PWA. The results are digitized and sent to the Main Board via opto couplers. The couplers provide patient isolation as well as serial data interface. The Main Board temporarily stores the data and routes it to the UI Board for display and/or printer. A reset signal to the SpO2 PWA is also provided so that the power up sequencing is corrected. If the SpO2 circuit quits communicating to the Main Board, the Main Board will attempt to reset the SpO2 PWA. 3.2.2 Cuff Blood Pressure (BP) and Pulse When the cuff and hose are attached to the ProCare Monitor and a NonInvasive Blood Pressure (NIBP) determination is initiated, the pump inflates the cuff. Pressure transducers PT1 and PT2 monitor pressure information. The pneumatic manifold has one valve, which is used to deflate the cuff. Valve control is through the Main Board. Once determinations are made for the systolic BP and diastolic BP, the Main Board calculates the pulse rate/ Mean Arterial Pressure (MAP). The results are then displayed on the UI Board and sent to the printer (if specified). The Pneumatic Valve/Manifold (PVM) device is controlled by the secondary processor. The secondary processor monitors pressure information from PT2. If an over-inflation condition occurs, the OVERPRESSURE signal is routed to the PVM to release the air pressure. The Main Board also generates an alarm condition with the speaker sounding and error code message on the UI Board. 3-4 ProCare Patient Monitor Service Manual 2008381-001 Revision B Principles of Operation: 3.3 Functional Description 3.2.3 Temperature (Model 200 and 400) The ProCare Monitor uses Alaris Turbo Temp technology to measure patient temperature. The Turbo Temp probe contains a heating element that preheats the probe to reduce determination time. The heating function is controlled by the Main Board. The Turbo Temp probe also contains a thermistor that indicates the temperature. When the probe is attached to the temperature connector and patient, the signal generated by the thermistor is routed to the Main Board. The Main Board converts the thermistor signal along with status information (i.e ORAL or RECTAL probe indicators) to a DIGITAL signal. The Main Board then processes the DIGITAL signal and displays the patient temperature on the UI Board and printer in Celsius or Fahrenheit. 3.2.4 Host Communication Port The Host Comm Port is used to interface the ProCare Monitor with other electronic devices (a central nurse's station or remote alarm device.) Signals can be sent to the ProCare Monitor to initiate blood pressure determinations and other functions. Patient data can also be retrieved through this port. For further information, reference the Dinamap ProCare Series Host Communication manual. 3.3 Functional Description The following paragraphs provide the functional interface relationship. The ProCare Monitor contains a number of electrical & electro-mechanical assemblies. These assemblies are: * Main Board PWA * User Interface (UI) Board PWA * SPO2 PWA (optional) * Printer (optional) * Pneumatic Valve/Manifold (PVM) * Optical Switch (optional) Revsion B ProCare Partient Monitor Service Manual 2009381-001 3-5 Principles of Operation: 3.3 Functional Description 3.3.1 Main Board PWA The ProCare Main Board is based on the Motorola MMC2107 integrated microprocessor. The microprocessor integrates Flash ROM, RAM, A/D converter with input multiplexor, SPI interface, and timers into one chip. This microprocessor is the primary processor for the ProCare Monitor. It services and controls the Patient Parameter Interface (PPI) devices, printer, UI Board, Real Time Clock, audio circuit, and host communication. The secondary processor controls the watchdog, pneumatic safety interlock, timing check, primary processor reset, and power supply control. The secondary processor is powered at all times. Independent software in the primary and secondary processor periodically communicate when the software systems are operating properly. When either system stops processing or detects an error, it stops communicating with the other. Either system, upon detecting a failure, can assert a safe state (herein called FAILSAFE) of the hardware. Upon entering a FAILSAFE condition, the Main Board will perform the following tasks: * Parameter monitoring disabled * Alarm tone sounding from speaker * Pneumatic FAILSAFE (deflate the cuff, pump off) * Normal communications interface disabled * Remote alarm control inactive * Hard keys except ON/OFF key inactive The ON/OFF key can reset the Monitor and end the FAILSAFE condition. The FAILSAFE condition will terminate automatically after 10 minutes to preserve battery power. All regulated DC power, isolated and non-isolated is generated on the Main Board from Battery supply. The external DC input is used to charge the battery via charging circuitry on the Main Board. 3.3.2 User Interface (UI) Board PWA The UI Board is used as a message center. It displays patient vital signs, alarms status, monitor set-up, limit violation, BP cycle and the time the data was received. The primary processor on the Main Board controls the UI Board. When the primary processor reads the parameter signals, it decodes the signals and routes the display information to the UI Board. The UI assembly also provides hardkey switches for the ProCare Main Board. The primary processor asserts a HIGH on the 16 outputs of the 1-of16 decoder/demultiplexer one at a time and then reads at the signal on SW_MUX. A LOW on SW_MUX indicates that the switch is asserted. 3-6 ProCare Patient Monitor Service Manual 2008381-001 Revision B Principles of Operation: 3.3 Functional Description 3.3.3 SPO2 PWA The ProCare monitor can be configured for use with either a Nellcor or Masimo SPO2 PWA. The SPO2 PWA provides continuous readings of oxygen saturation and pulse rate. Additional circuitry on the Main Board provides power, data communications, and isolation between SPO2 PWA and primary processor. Patient data received from the finger sensor is filtered, amplified, and analyzed on the SPO2 PWA. The information is sent to the Main Board via the optically coupled electrically isolated serial connection. The primary processor receives the data and routes it to the UI board for display. The data is also sent to the printer if specified. 3.3.4 Printer The printer receives power from the Main Board and communicates with the primary processor. Printer presence and print head temperature is indicated by PR_TH signal to the primary processor. When a print command is sent to the printer from primary processor, the following will occur: * PR_CLK signal - transfer the data into print head * PR_DI signal - serial dot to be printed * PR_LAT signal - latch the data stream into the head * PR_ST1-6 - cause the head to print various sections * PR_M1-4 signals - control power sequentially to the two stepper motor windings Together these signal (CONTROL DATA) cause the printer to print a graphic hardcopy of the patient vital sign values and trend data. It also causes the printer to print a hardcopy of error logging and service record data. The printer has a built-in sensor to monitor the printer paper presence. When the printer is out of paper, it sends a PAPER OUT signal to the primary processor. 3.3.5 Pneumatic Valve/Manifold (PVM) The PVM assembly consists of a pump, a deflate valve, and a dump valve. The PVM inflates/deflates the cuff during BP determinations. During normal operation the PVM is controlled by the primary processor. If a failsafe mode or overpressure condition occurs, the secondary processor provides the appropriate control signals to insure a safe condition, where the cuff vents to ambient atmosphere pressure. 3.3.6 Optical Switch The optical switch indicates whether the temperature probe is inserted in the probe holder or not. The Main Board powers the switch. Revsion B ProCare Partient Monitor Service Manual 2009381-001 3-7 Principles of Operation: 3.3 Functional Description 3-8 ProCare Patient Monitor Service Manual 2008381-001 Revision B Optical Switch Main Board SpO2 Probe (Optional) SpO2 Circuit Isolated DC Power Supply Temp Probe (Optional) Pump/Valve Manifold Temp Control/ Data External DC 6V Battery Host Comm Port (rear) Tubing Control PT1/ PT2 NIBP Data Pneumatic Control UI Board Temp Control Interface Regulated DC Power Control / Data RS-232 Switches Display LEDs Real Time Clock Remote Alarm Audio Control Printer Driver Speaker Printer Control/ Data BP Cuff tubing ProCare Vital Signs Monitor System Block Diagram (1 of 2) page 3-9/10 Real Time Clock External DC 6Volt Battery Isolated Power Supply Regulated DC Power Supply Printer Drivers Audio Control Speaker Pneumatic Valve Control PT1/PT2 NIBP Data SpO 2 Interface Host Communications Temp Probe (optional) Temperature Control/Data Temperature Control ProCare Vital Signs Monitor Main Board Diagram (2 of 2) page 3-11/12 4 Revision B Calibration & Maintenance ProCare Patient Monitor Service Manual 2009381-001 4-1 For your notes 4-2 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.1 Introduction 4.1 Introduction This section contains general Monitor service procedures, including alarm code interpretation, service mode operation, and periodic maintenance and battery care. Refer to Section 5 for disassembly and reassembly procedures and related component service information. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-3 Calibration & Maintenance: 4.2 Configuring Your ProCare Monitor 4.2 Configuring Your ProCare Monitor 4.2.1 Unpacking and Preparation for Installation 1.Unpack and identify the contents of all shipping materials. 2.Remove the ProCare monitor. 3.Unpack the external DC Power Input. 4.Insert the external DC Power Input connector into the DC input on the lower right side of the monitor. 5.Plug the DC Power Input transformer into a Hospital Grounded AC receptacle. The word CHARGING will illuminate green on the front of the monitor indicating that an external power source is available. Prior to usage, it is necessary to charge the monitor for 12 hours. 4.2.2 Set the Date and the Clock Setting the Date and Time To set the date and time on the ProCare Monitor, you must first access the configuration mode. The following table illustrates the menus and the corresponding LCD display graphics. Setting SpO2 Averaging Window Text in Window Pulse Rate and SpO2 SpO2 Temp Unit of Measure Temperature Year Systolic Month MAP/Cuff Day Diastolic Hour min Minute min Procedures 1. With the Monitor off, press and hold the Menu button at the same time as pressing the On/Off button for 3 seconds. 4-4 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.2 Configuring Your ProCare Monitor 2. The Monitor automatically switches on in configuration mode. 3. To set the date and time, press the Menu button to move from one setting to another. (You will need to press the Menu button several times until the year setting appears. Use the +/- buttons to increment or decrement the individual settings.) Once you are finished changing a setting, press the Menu button again to move to the next setting. Note: For the date and time to be saved, you must advance the menu through the min setting. 4. To exit config mode, press the On/Off button. 4.2.3 Parameter Level Functional Testing After the initial configuration is complete, perform functional testing of each of the parameters, using the accessories supplied with the ProCare Monitor. Refer to the ProCare Operator's Manual for more detailed parameterspecific instructions. Perform a blood pressure by connecting the supplied hose and cuff together, then attaching to the front of the ProCare Monitor. Press the Inflate/Stop on the front to begin the NIBP cycle. Connect the supplied temperature probe to it’s corresponding connection. A predictive temperature will begin once the probe is removed from its holster. Replace the probe after completion of the Temp cycle. ·The SpO2 sensor is an assembly consisting of two parts: the DS-100A, and the extender cable DOC-10. Connect the cables prior to attaching to the monitor. An SpO2 reading will be displayed within moments of attaching the sensor to either a Nellcor simulator or to your finger. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-5 Calibration & Maintenance: 4.3 Periodic Maintenance 4.3 Periodic Maintenance 4.3.1 As Required Perform the following maintenance procedures as required. 4.3.1.1 Integrity of Hoses and Cuffs When the pneumatic integrity of any NIBP cuff and hose is in doubt, replace the cuff and hose, and discard the questionable accessories. 4.3.1.2 Cleaning of Monitor CAUTION: Do not clean Monitor with isopropyl alcohol or other solvents. Wipe the exterior of the Monitor with a cloth slightly dampened with mild detergent or normal hospital bactericides. Use dishwashing detergents such as IVORY and JOY (registered trademarks of Procter & Gamble Corp.), or PALMOLIVE (registered trademark of Colgate-Palmolive Corp.) Do not immerse unit. 4.3.1.3 Cleaning of Accessories Clean the adult cuffs supplied for use with the monitor by hand washing in warm, soapy water. However, take care to avoid entry of water into the cuff and hoses at any time. If water enters the cuff, dry the cuff by passing air through it. The neonatal cuffs are for single patient use - discard if they become soiled. Clean cuffs and hoses with a cloth slightly dampened with mild detergent. Do not immerse hoses. Do not immerse cuffs without prior application of cuff hose caps. Clean SpO2 sensor surface before and after each patient use. Clean SpO2 sensor with a cloth slightly dampened with a mild detergent. Wipe SpO2 sensor to ensure all detergent residue has been removed. Compatible cleaning and disinfecting solutions are: Chlorine bleach disinfectant, 5.25%, 0.75 cup per gallon of water. CAUTION: Do not apply isopropyl alcohol to the Monitor - some parts can become marred and cracked. 4-6 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.3 Periodic Maintenance Cidex Formula 7 (registered trademark of Johnson & Johnson Medical Products, Inc.) or pHisoHex (registered trademark of Winthrop-Breon Laboratories.) Quaternary-based germicidal detergents like VESTAL INSURANCE (registered trademark of the Vestal Corp.), HI-TOR PLUS (registered trademark of the Huntington Corp.), or VIREX (registered trademark of S.C. Johnson & Son Corp.) For the above, follow manufacturers' recommendations for dilution rate and use. These recommendations are not an endorsement of the manufacturers or of the effectiveness of these materials for cleaning or disinfecting. 4.3.1.4 Long-Term Storage If it becomes necessary to store the Monitor for an extended period of time, remove all attached accessories. Attach the original packing inserts, and place the monitor into the original shipping container. Long-term storage of a discharged lead-acid battery can permanently degrade its storage capacity. Therefore, the battery should be fully charged before storage. Batteries in storage should be charged every six months to maintain their capacity. Elevated temperatures will shorten battery life and can lead to permanent battery damage. 4.3.2 Annual Procedures Perform the test procedures described in section 4.6 every twelve months, or whenever the accuracy of of the monitor is in doubt. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-7 Calibration & Maintenance: 4.4 Care of the Storage Battery 4.4 Care of the Storage Battery The Monitor uses one Lead-Acid storage battery. The battery can be charged at any time without reducing the charging capacity. 4.4.1 Battery Charging The Monitor charges the Lead-Acid battery whenever the AC power supply is in use. The Monitor automatically senses if the battery needs recharging. Battery charging will continue as long as the Monitor is connected to the AC power supply, even when the Monitor is turned off. ·Batteries should be charged before first use or after long periods of storage. ·The battery should be charged for 8 hours before use, as a charged battery loses some charge when left in storage. It is possible to use the Monitor while the Monitor is charging. ·The battery should be charged at room temperature (59° F - 86° F; 16° C 30° C). ·It is normal for the battery to become warm during charging or after use. ·Batteries can be charged or topped-off at any time. It is not necessary to wait until they are fully discharged. ·If the monitor is idle for extended periods, it should be fully charged once a month to ensure optimum performance. 4-8 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.5 Safety Testing 4.5 Safety Testing To adequately test the safety and integrity of the ProCare Monitor, the following test equipment is recommended: • 12VDC Power Supply • DMM (Fluke 8842 or equivalent) • NIBP Analyzer (DNI Nevada "Cufflink" or Equivalent) • Adult BP Cuff, Neonate BP Cuff, hose, inflation bulb, and mandrel • Inflation bulb and associated tubing • Calibration Kit, p/n 320246 available through GE Medical Systems • SPO2 Simulator (for appropriate SPO2 type if SPO2 is installed) • SPO2 Cable (for appropriate SPO2 type, if SPO2 is installed) • TE 1811 Temperature Probe Simulator (if TEMP is installed.) The Temperature Simulator for the Alaris System is available from Alaris Medical Systems, Inc. (619) 458-7000 • Printer Paper (if PRINTER is installed) CAUTION! Calibration equipment should always be kept dry and free of particulate matter. Moisture or foreign substances introduced to the pneumatic system will likely cause damage to the monitor and/or the accessories. Complete the Test Record (Appendix A) as tests are performed. Note: This test is written so that a knowledgeable technician who is familiar with the ProCare monitor and the test equipment and will be able to follow the test procedure. Note: To enter service mode press and hold the CYCLE buttons while pressing the ON/OFF button for 3 seconds. Notes on Electrical Safety Testing of the ProCare Monitor: The DINAMAP® ProCare Monitor is designed and tested to meet electrical safety standard IEC 601-1. Requirements in this standard parallel requirements in NFPA99 relating to electrical safety. This product meets the requirements of NFPA99 section 7-5.1.2.2. (grounding of appliances) through the use of double insulation in the external power supply module (“Power Brick”.) The absence of a ground connection on the power brick obviates the need for any ground resistance test, such as that described in NFPA99 section 75.1.3.2. (Some non-USA Power bricks are equipped with a non-conductive Revision B ProCare Patient Monitor Service Manual 2009381-001 4-9 Calibration & Maintenance: 4.5 Safety Testing pin at the ground pin location to aid mechanical retention of the Power Brick into the receptacle.) Dielectric strength (“Hi-Pot”) testing of the double (reinforced) mains insulation (4000 V RMS, 60 Hz) may be done by disconnecting the Power Brick from the Monitor and applying the Hi-Pot tester as follows: Connect one lead to the two mains power prongs. Connect the other lead to the conductive sleeve on the DC output connector at the end of the cord. Hi-Pot testing of the patient connections (1500 VRMS, 60 Hz) may be done by connecting one lead of the Hi-Pot tester to the outer shell of a spare DC connector plug inserted into the DC input connector on the Monitor. The other lead of the tester should be connected to the patient connection being tested. WARNING: Don’t electrocute yourself or anyone else when performing HiPot testing. NOTE: Hi-Pot testing is done on every unit at the factory and should not be repeated unnecessarily nor performed more often than required. 4.5.1 Temp Circuit Leakage Test 1. Setup an IEC 601-1 approved leakage tester to apply 240 VAC to an isolated circuit. 2. Plug temp probe Hi Pot adapter into the temp jack. 3. Record and verify the temp circuit leakage current. 4.5.2 SpO2 Circuit Leakage Test 1. Setup an IEC 601-1 approved leakage tester to apply 240 VAC to an isolated circuit. 2. Plug an SpO2 cable into the SpO2 connector on the front of the unit. 3. Plug SpO2 probe Hi Pot adapter in to the DB9 jack at the end of the SpO2 cable. 4. Record and verify the SpO2 circuit leakage current. 4-10 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 4.6 ProCare Patient Monitor Parameter Tests Complete the Test Record (Appendix A) as tests are performed. Note: This test is written so that a technician who is familiar with the ProCare monitor and the test equipment will be able to successfully complete the test procedures. To enter service mode press and hold the CYCLE button while pressing the ON/OFF button for 3 seconds. Saving Changes into Flash Memory: Modifications made to the Monitor’s settings/configuration must be saved prior to placing the Monitor into service. The Monitor must be in service mode for any changes to be saved. Events that require you to manually save to the Monitor’s memory include calibration attempts and enabling/disabling the Monitor’s parameters (refer to section 4.6.4.) It is not necessary to manually save following a pressure transducer calibration verification or simple calibration check. The number of times the flash memory can be saved is limited. The fatal alarm error 975 may be issued after this process is performed more than 90 times. Following issuance of this fatal alarm, the monitor must be returned to the GE Medical Systems Information Technologies Service Center to have the flash memory reset so that future saves can be performed. 4.6.1 SETUP 1. Connect your NIBP Analyzer to the ProCare Monitor. 2. ‘T’ an inflation bulb into the pneumatic setup 3. Consult the following diagram for pneumatic setup guidelines. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-11 Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 4.6.2 Leakage Testing Note: To enter service mode press and hold the CYCLE button while pressing the ON/OFF button 1. Turn the Monitor ON and enter Service Mode. 2. Press CYCLE button and the min display should change to a 1 3. Close the valve on the inflation bulb 4. Set the NIBP Analyzer to "Leak test" function 5. Press the "Pump" button on the NIBP Analyzer 6. After the system inflates to 200mmHg, wait for the "Wait" on the NIBP analyzer message to be cleared 7. Press the "Start" button on the NIBP Analyzer to begin the leakage test 8. After 60 seconds the NIBP Analyzer will display the leakage rate 9. Record and verify the leakage rate. 10. Turn the ProCare Monitor off 4.6.3 Pressure Transducer Verification Note: To enter service mode press and hold the CYCLE button while pressing the ON/OFF button 1. Turn the Monitor ON and enter Service Mode. 2. The HISTORY LED should display 0. 3. Set NIBP Analyzer to "Manometer" function and press "Zero Pressure" 4. Press CYCLE button, HISTORY display should change to a 1. 5. Use the inflation bulb to inflate the cuff, hose and pressure indicator setup to 200mmHg 6. Record and verify the pressure reading on the top LED display (SYSTOLIC) 7. Record and verify the pressure reading on the bottom LED display (DIASTOLIC) 8. Use the valve on the bulb to reduce pressure to 150mmHg 9. Record and verify the pressure reading on the top LED display (SYSTOLIC) 10. Record and verify the pressure reading on the bottom LED display (DIASTOLIC) 11. Use the valve on the bulb to reduce pressure to 100mmHg 4-12 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 12. Record and verify the pressure reading on the top LED display (SYSTOLIC) 13. Record and verify the pressure reading on the bottom LED display (DIASTOLIC) 14. Use the valve on the bulb to reduce pressure to 50mmHg 15. Record and verify the pressure reading on the top LED display (SYSTOLIC) 16. Record and verify the pressure reading on the bottom LED display (DIASTOLIC) If any of the tests fail, continue to section 4.6.4. Otherwise, continue to section 4.6.5 4.6.4 Pressure Transducer Calibration Perform only if Pressure Transducer Verification is out of tolerance as specified in Appendix A. Note: To enter service mode press and hold the CYCLE button while pressing the ON/OFF button. 1. Turn the ProCare Monitor ON and enter Service Mode. 2. The min window should display 0. 3. Open valve on bulb to open pressure system to atmosphere 4. Set NIBP Analyzer to "Manometer" function and press "Zero Pressure" 5. Press CYCLE button until the min window shows 1. 6. Close valve on bulb and manually inflate pressure to 200 mmHg (using the CuffLink manometer as reference). 7. Press MENU button when pressure reads 200 mmHg to save calibration setting. 8. Two steps are necessary for burning changes into flash: a. Press the CYCLE button repeatedly until the number 6 is displayed in the min window. Note: the number of remaining changes to the flash memory available are displayed in the MAP/Cuff window. Following each true calibration, the Monitor decrements by 1 the number of remaining calibrations available. If the MAP/Cuff window is displaying a figure of less than 10 and the accuracy of the Monitor is in doubt, contact General Electric Medical Systems Information Technologies Technical Support at 1-877-274-8456. b. Press and hold the MENU button until two beeps are heard (one when Revision B ProCare Patient Monitor Service Manual 2009381-001 4-13 Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests the button is pressed and another after the button has been depressed long enough for the process to complete.) 9. Turn the ProCare Monitor off 4.6.5 Overpressure Verification Note: To enter service mode press and hold the CYCLE button while pressing the ON/OFF button. 1. Remain in service mode. 2. Use the inflation bulb to inflate pressure until valve opens. 3. Record and verify pressure at which valve opens. 4. Press CYCLE button so that the min window changes to a 2. 5. Use the inflation bulb to inflate pressure until valve opens. 6. Record and verify pressure at which valve opens. 7. Turn unit off. 4.6.6 Button Testing 1. Turn the Monitor on. 2. Record software revision as shown in the systolic and diastolic display. 3. Press START/STOP button. 4. Verify an NIBP determination has been initiated. 5. Block pump port and verify "E80" alarm. 6. Press SILENCE button, verify alarm has been silenced. 7. Verify flashing red indicator of Silence. 8. Press the SILENCE button, verify alarm condition is removed 9. Press ALARM button several times, verify unit cycles through all alarm settings (i.e SYS, DIA, SPO2). 10. Turn the Monitor off 4-14 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 4.6.7 LED Tests 1. Power on the ProCare Monitor. 2. During the power-up self-test verify all 7 segment LED display segments and all discrete LEDs (except CHARGING LEDs) illuminate and are the correct color. LED COLOR MATRIX RED YELLOW GREEN NOT LIT Systolic Pulse Rate AUTO CYCLE CHARGING Diastolic LOW HISTORY °F BATTERY °C BATTERY ICON HIGH (all) ALARM VOLUME LOW (all) PULSE VOLUME Alarm Bell Icon INFLATE PRESSURE SpO2 Strength Meter 3. Repeat power up cycle until all LEDs are checked. 4.6.8 External DC Verification 1. Plug the DC power cable into the monitor. 2. Verify that the CHARGING indicator is illuminated. 4.6.9 NIBP Determination 1. Set NIBP Analyzer to Adult Mode: SYS/DIA = 120/80 MAP = 90 BPM = 80 2. Press START/STOP button on the Monitor to begin determination 3. Record and verify systolic, diastolic, map and heart rate from the monitor display 4. Press CYCLE button to initiate a determination in "Auto BP" mode. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-15 Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 5. Record and verify systolic, diastolic, map and heart rate from the monitor display 6. Press CYCLE button until is displayed on the HISTORY LED to initiate a determination in STAT mode. 7. Record and verify systolic, diastolic, map and heart rate from the monitor display 8. Press Start/Stop button, end STAT mode. 4.6.10 NIBP Overpressure Verification 1. Restrict airflow through cuff hose port. 2. Press "Inflate/Stop" to begin NIBP determination 3. Verify that alarm sounds is displayed on the SYSTOLIC display and an audible 4. Remove the air restriction 5. Press "Inflate/Stop" and verify that the pump does not start 6. Press the "Silence" button 7. Press the "Silence" button again 8. Verify the alarm condition is cleared from the SYSTOLIC display 4.6.11 Temperature (Perform if equipped with Temp module) The Temperature Simulator for the Alaris System is available from Alaris Medical Systems, Inc. (619) 458-7000. 1. Disconnect the temp probe from the Monitor. 2. Connect the probe simulator to the Monitor. 4. Set the probe simulator to 80.2° F. 5. Record and verify the reading in the TEMP display is 80.2° F ±0.2° F. 6. Set the probe simulator to 102° F. 7. Record and verify the reading in the TEMP display is 102° F ±0.2° F. 8. Set the probe simulator to B.P. and verify reading is 106.0° F ± 0.2° F. Press broken probe button down and verify the Monitor displays 4-16 ProCare Patient Monitor Service Manual 2009381-001 . Revision B Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 9. Calibration verification is complete. Disconnect the probe simulator and install the temperature probe. If the Monitor does not pass the calibration verification, then the Monitor needs repair. 4.6.12 SpO2 (Perform only if equipped with SpO2 module) Note for Monitors equipped with Nellcor SpO2: Nellcor is aware that various Nellcor oximetry platforms have some interesting behaviors when used with various pulse simulators. On occasion when testing the integrity of the Nellcor oximetry system, abnormal results may occur when introducing large changes in the pulse rate and/or pulse amplitude. Extreme changes in rate sent to the Nellcor sensor by the SpO2 simulator may cause the SpO2 algorithm to completely miss in finding the pulse rate. This is an expected result. To workaround this, incrementally step up or down the settings on your SpO2 simulator and allow for the Monitor to detect and display the new pulse rate or saturation. Nellcor recommends use of the SRCmax Portable Tester for use with ProCare Monitors equipped with the Nellcor SpO2 system. Masimo recommends BIO-TEK SpO2 simulators. 1. Connect the appropriate SpO2 simulator and cable to the SpO2 connector. 2. Verify the unit displays a: Pulse value Saturation value Signal Strength Bar Graph 3. Disconnect the SpO2 cable 4. Verify the unit generates a alarm and speaker is sounding 5. Press the SILENCE button. 6. Verify the sound has stopped but the error display remains 7. Re-connect the SpO2 sensor 8. Verify the unit displays a: Pulse Value Saturation value Signal Strength bar Graph Revision B ProCare Patient Monitor Service Manual 2009381-001 4-17 Calibration & Maintenance: 4.6 ProCare Patient Monitor Parameter Tests 4.6.13 Printer Output Test 1. Load Thermal Paper into the print mechanism 2. Press PRINT button 3. Verify the printer outputs a record and print quality is good 4.6.14 Communication Port Test 1. Connect unit to a PC terminal emulator. 2. Turn unit on. 3. Type " NC0!E", press "Enter" 4. Verify response from unit " NC+!@" 5. Verify that within 40 seconds the pump starts. 6. Type " ND!5", press "Enter" 7. Verify response from unit " ND+!A" 8. Verify the pump stops. 4-18 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: 4.7 Alarm Code Interpretation 4.7 Alarm Code Interpretation If any other alarms appear that are not listed in the paragraphs that follow, record the error message and report the failure to Customer Support. Refer to the Operation Manual for information about patient alarms and general procedural alarms. 4.7.1 System Failures When a system failure is encountered, the error code is displayed on the screen for five seconds and the system enters failsafe mode. The error code is logged in the history log. General system error codes are listed below. Alarm Conditions and Error Codes When responding to a Monitor alarm, always CHECK THE PATIENT FIRST and then check the Monitor, cuff, hose and sensors. Press SILENCE to reset patient alarm conditions. NIBP Alarm Definition Possible Cause E89 NIBP No Determination Unable to make an NIBP determination due to insufficient signal. E84 Timeout Determination time > 2 minutes. Motion Artifact. E85 Timeout One cuff pressure at > 1 minute. Motion artifact. E83 Timeout: Inflation Inflation time is > 40 seconds Possible air leak is being detected. E82 Excess Air in Cuff Residual air in cuff above threshold for successful auto-zero. E80 Overpressure Overpressure condition detected Definition Possible Cause E20 SpO2 No Sensor SpO2 sensor not connected. No sensor code detected. Sensor failure E21 SpO2 Replace Sensor SpO2 sensor or cable possibly defective. Cable not connected properly. SpO2 Alarm Revision B ProCare Patient Monitor Service Manual 2009381-001 4-19 Calibration & Maintenance: 4.7 Alarm Code Interpretation E23 SpO2 Sensor off finger No SpO2 signal, check or reposition the sensor E25 SpO2 No Signal No or very low SpO2 signal, reposition sensor Temperature / Printer / Miscellaneous Alarm 4-20 Definition Possible Cause E63 Temp Disconnected or Wrong Probe Type Incorrect type of temperature probe: use TurboTemp-type temperature probe E61 Temp Probe Broken Bad temperature probe Temp probe not properly connected E66 Temp probe Too Hot Bad temperature probe Verify monitor with known good accessories E10 Printer No Paper Printer is out of paper Printer door open Printer may be defective E11 Printer Too Hot E12 Recorder cannot print Main battery voltage is too low to operate E00 Memory Lost Refer Monitor to Customer Service 900999 Internal Memory Errors Refer Monitor to Customer Service ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix A - Test Results Form Appendix A - Test Results Form Step 4.6.2 Description Min Max 0 6 Pressure reading at 200mmHg, top display - Systolic 197 203 Pressure reading at 200mmHg, bottom display - Diastolic 197 203 Pressure reading at 150mmHg, top display - Systolic 147 153 Pressure reading at 150mmHg, bottom display - Diastolic 147 153 Pressure reading at 100mmHg, top display - Systolic 97 103 Pressure reading at 100mmHg, bottom display - Diastolic 97 103 Pressure reading at 50mmHg, top display - Systolic 47 53 Pressure reading at 50mmHg, bottom display - Diastolic 47 53 4.6.5 Overpressure threshold, Adult (mmHg) 305 325 Overpressure threshold, Neonate (mmHg) 155 159 4.6.6 on off 107 133 Pass-Fail-N/A Leakage Leakage Result (mmHg) 4.6.3 Actual Pressure Transducer Verification Overpressure Verification Buttons Software revision in systolic display (top) Software revision in systolic display (bottom) NIBP alarm initiated "E80" displayed on SYSTOLIC display Audible alarm can be silenced "Silenced" LEDs flash Overpressure alarm can be cleared Alarm button is functioning 4.6.7 Display All 7-Segment LEDs Light Correct Color All Discrete LEDs Light, Correct Color 4.6.8 4.6.8 External DC Detection Charging indicator LED illuminated 4.6.9 NIBP Determination Systolic reading (mmHg) Revision B ProCare Patient Monitor Service Manual 2009381-001 4-21 Calibration & Maintenance: Appendix A - Test Results Form Step 4.6.9 4.6.10 Description Min Max Diastolic reading (mmHg) 67 93 MAP reading (mmHg) 85 95 Heart rate reading (bpm) 76 84 Systolic reading (mmHg) 107 133 Diastolic reading (mmHg) 67 93 MAP reading (mmHg) 85 95 Heart rate reading (bpm) 76 84 Systolic reading (mmHg) 107 133 Diastolic reading (mmHg) 67 93 MAP reading (mmHg) 85 95 Heart rate reading (bpm) 76 84 Temperature Test 80.0°F 80.4°F Temperature reading at 80.2° F 79.9° F 80.5° F Temperature reading at 102.0° F 101.8° F 102.2° F N/A N/A Actual Pass-Fail-N/A NIBP Determination (continued) NIBP Overpressure "E80" displayed on SYSTOLIC display Pump will not start Overpressure alarm can be cleared 4.6.11 Temperature reading at BP verify reads E61 4.6.12 SpO2 Pulse Value Displayed Saturation Value Displayed Signal Strength Bar Graph Displayed "E23" displayed on SpO2 display Alarm is silenced, error display remains Pulse Value Saturation Value Displayed Signal Strength Bar Graph Displayed 4.6.13 Printer Test Printout is generated cleanly 4.6.14 Communication Port Test "_NC+!@" is displayed on terminal Pump stops 4-22 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix B - Connectivity Appendix B - Connectivity CHANT • Provides HL7 output for electronic Patient Medical Records • Uses ILCs (Isolated Level Convertors) where required along with the CHANT software to communicate with the HIS System Compatible Monitors: DINAMAP XL, Compact, MPS (Select and Portable), PRO 100-400 series, ProCare Series, and PRO 1000. Nurse Call System • When the DINAMAP monitor alarms, the Nurse Call System is triggered. • Uses a Nurse Call Cable to attach to the System. P/N 487208CR. Compatible Monitors: Compact, PRO 100-400 Series, ProCare Series, and PRO 1000. Alarm View • Wireless transmitter attached to the Monitor. • Sends alarms and vital signs results to a pager Compatible Monitors: Compact, MPS (Select and Portable) PRO 100-400 series, ProCare Series, and PRO 1000. CIC • Provides Central Alarm Notification & Data Management. • Compatible with StatView for paging. Compatible Monitors: PRO 1000 Version 2 and Dash 2000 (both hardwired) ApexPro • Provides Central Alarm Notification and data management. • Transmits data wirelessly. • Pro 100-400 Series and ProCare (requires P/N IPC-1931) PatientNet • Provides central alarm notification and data management. • Lethal arrhythmia at the Central Station. • WMTS frequency hopping spread spectrum. Note: Dash 2000 is incompatible with PatientNet. ILC-1926 • Interfaces the PRO 100-400 series or ProCare monitor to ANY hardwired connection. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-23 Calibration & Maintenance: Appendix B - Connectivity • Requires Cables: -PRO 100-400, ProCare Series, Compact, Plus: cable p/n 683235 -XL monitor series: p/n 682234 ILC-1927 • Interfaces the PRO 1000 monitor to any hardwire connection. • Installs into the Communications bay at the rear of the monitor. IPC1928 • Interfaces the PRO 1000 Monitor to the Unity network, hardwire. • Installs into the Communications bay at the rear of the monitor. IPC1931 Connects DINAMAP Monitors to the CIC system. Transfers vital signs results from the Monitor to the CIC Requirements for use: - Plug DINAMAP into ApexPro using the DINALINK cable. 4-24 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix B - Connectivity - Apex version 2 - CIC version 3 Revision B ProCare Patient Monitor Service Manual 2009381-001 4-25 Calibration & Maintenance: Appendix C - Display Cover: Removal, Installation Appendix C - Display Cover: Removal, Installation To remove the plastic display cover: 1. Place the Monitor on a stable surface. 2. Insert a flat-head screwdriver between the top of the display cover and the body of the monitor. 3. Twist the screwdriver so that the display cover is slightly separated from the body of the monitor. 4. Using two hands, grasp the top-middle edge of the display cover and pull away and down. 4-26 ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix C - Display Cover: Removal, Installation 5. The fascia and displays are now visible. Installing the Display Cover: 1. Place the Monitor on a flat, stable surface. 2. Insert the tabs at the base of the display cover into the slots underneath the bottom row of LEDs. 3. Using two hands, apply firm pressure to the outside edges, while flexing the upper middle-edge of the display cover away from the monitor. It is imperative that even pressure is applied as the plastic retention tabs can break free from the display cover. 4. Apply steady, even pressure to the opposite sides of the display cover until an audible click is heard. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-27 Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies Appendix D: Replacement Parts and Assemblies ID numbers reference the explode drawings in Section 5. ID FRU Number Description Content/comments 1 2009910-001 Main PWA Supplied with Arterial ref product s/w 2 2009911-001 Main PWA- Ausc Supplied with Auscultatory version of Monitor 3 2009912-001 UI PWA 4 2009913-001 Nellcor Module Supplied with mounting spacers. 5 2009914-001 Masimo Module Supplied with mounting spacers. 6 2009915-001 Plastics kit 1. 2. 3. 4. 5. 6. 7. 8. 9. 2009916-001 Screw kit All internal hardware, screws, washers etc 7 2009918-001 Temperature 1. Housing front & rear. 2. Mounting bracket and plastic guide. 3. Sensor and cable. 4. Mounting hardware 8 2009917-001 Printer 1. Printer 2. Printer chassis and screw. 3. Printer door with roller and label. 9 2009919-001 Printer door Door with label and roller 10 2009920-001 Pneumatics Pump assembly 11 2009921-001 Dump valve Dump valve and adhesive tape to apply. 12 2011645-001 Kit, Keypads Set, left and right keypads 13 2009922-001 Speaker Include mounting bracket 14 2009923-001 Host comms cable With mounting h/w 2008538-001 Power brick UK UK plug 240V input 2008539-001 Power brick EUR Euro plug 230V input 2009460-001 Power brick US US plug 120V input 2010429-001 2010478-001 2010479-001 2010480-001 English 400 lbl kit English 300 lbl kit English 200 lbl kit English 100 lbl kit 1. keypad labels set (LH & RH) 2. Fascia 3. Display cover For language variants reference following pages Help card guides All 3 guides and for each language 15 16 4-28 Case, front & rear Handle front & rear with h/w Bulkheads 4 types & NIBP insert. Nellcor & Masimo ‘inside’ labels Std. labels, DC input, No temp side label. Battery door & foam. Help card guide and rubber feet. Printer door (and blanking plate when available). Cable tie hardware ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies 2010429-001 Label Kit English 400 printer P/N (-001) Language Model Is Printer Installed? 2010429-001 English 400 Printer Installed Traditional 2010478-001 English 300 Printer Installed Traditional 2010479-001 English 200 Printer Installed Traditional 2010480-001 English 100 Printer Installed Traditional 2010481-001 English 400 Printer Installed Auscultatory 2010482-001 English 300 Printer Installed Auscultatory 2010483-001 English 200 Printer Installed Auscultatory 2010484-001 English 100 Printer Installed Auscultatory 2010485-001 English 400 Traditional 2010486-001 English 300 Traditional 2010487-001 English 200 Traditional 2010488-001 English 100 Traditional 2010489-001 English 400 Auscultatory 2010490-001 English 300 Auscultatory 2010491-001 English 200 Auscultatory 2010492-001 English 100 Auscultatory 2010493-001 French 400 Printer Installed Traditional 2010494-001 French 300 Printer Installed Traditional 2010495-001 French 200 Printer Installed Traditional 2010496-001 French 100 Printer Installed Traditional 2010497-001 French 400 Printer Installed Auscultatory 2010498-001 French 300 Printer Installed Auscultatory 2010499-001 French 200 Printer Installed Auscultatory 2010500-001 French 100 Printer Installed Auscultatory 2010501-001 French 400 Traditional 2010502-001 French 300 Traditional Revision B Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 4-29 Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies 2010503-001 French 200 Traditional 2010504-001 French 100 Traditional P/N (-001) Language Model 2010505-001 French 400 Auscultatory 2010506-001 French 300 Auscultatory 2010507-001 French 200 Auscultatory 2010508-001 French 100 Auscultatory 2010509-001 German 400 Printer Installed Traditional 2010510-001 German 300 Printer Installed Traditional 2010511-001 German 200 Printer Installed Traditional 2010512-001 German 100 Printer Installed Traditional 2010513-001 German 400 Printer Installed Auscultatory 2010514-001 German 300 Printer Installed Auscultatory 2010515-001 German 200 Printer Installed Auscultatory 2010516-001 German 100 Printer Installed Auscultatory 2010517-001 German 400 Traditional 2010518-001 German 300 Traditional 2010519-001 German 200 Traditional 2010520-001 German 100 Traditional 2010521-001 German 400 Auscultatory 2010522-001 German 300 Auscultatory 2010523-001 German 200 Auscultatory 2010524-001 German 100 Auscultatory 2010525-001 Spanish 400 Printer Installed 2010526-001 Spanish 300 Printer Installed 2010527-001 Spanish 200 Printer Installed 2010528-001 Spanish 100 Printer Installed 2010529-001 Spanish 400 2010530-001 Spanish 300 2010531-001 Spanish 200 4-30 Is Printer Installed? Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies 2010532-001 Spanish 100 P/N (-001) Language Model Is Printer Installed? 2010534-001 Italian 400 Printer Installed 2010535-001 Italian 300 Printer Installed 2010536-001 Italian 200 Printer Installed 2010537-001 Italian 100 Printer Installed 2010538-001 Italian 400 2010539-001 Italian 300 2010540-001 Italian 200 2010541-001 Italian 100 2010542-001 Swedish 400 Printer Installed 2010543-001 Swedish 300 Printer Installed 2010544-001 Swedish 200 Printer Installed 2010545-001 Swedish 100 Printer Installed 2010546-001 Swedish 400 2010547-001 Swedish 300 2010552-001 Swedish 200 2010553-001 Swedish 100 2010554-001 Dutch 400 Printer Installed 2010555-001 Dutch 300 Printer Installed 2010556-001 Dutch 200 Printer Installed 2010557-001 Dutch 100 Printer Installed 2010588-001 Dutch 400 2010559-001 Dutch 300 2010560-001 Dutch 200 2010561-001 Dutch 100 2010578-001 Chinese 400 Printer Installed 2010589-001 Chinese 300 Printer Installed 2010590-001 Chinese 200 Printer Installed 2010592-001 Chinese 100 Printer Installed 2010593-001 Chinese 400 Revision B Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 4-31 Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies 2010594-001 P/N (-001) Chinese Language 300 Model Is Printer Installed? 2010595-001 Chinese 200 2010596-001 Chinese 100 2010597-001 Japanese 400 Printer Installed 2010598-001 Japanese 300 Printer Installed 2010599-001 Japanese 200 Printer Installed 2010600-001 Japanese 100 Printer Installed 2010601-001 Japanese 400 2010602-001 Japanese 300 2010603-001 Japanese 200 2010604-001 Japanese 100 2010605-001 Danish 400 Printer Installed 2010606-001 Danish 300 Printer Installed 2010607-001 Danish 200 Printer Installed 2010608-001 Danish 100 Printer Installed 2010609-001 Danish 400 2010610-001 Danish 300 2010611-001 Danish 200 2010612-001 Danish 100 2010613-001 Norwegian 400 Printer Installed 2010614-001 Norwegian 300 Printer Installed 2010615-001 Norwegian 200 Printer Installed 2010616-001 Norwegian 100 Printer Installed 2010617-001 Norwegian 400 2010618-001 Norwegian 300 2010619-001 Norwegian 200 2010620-001 Norwegian 100 2010621-001 Korean 400 Printer Installed 2010622-001 Korean 300 Printer Installed 2010623-001 Korean 200 Printer Installed 4-32 Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies P/N (-001) Language Model Is Printer Installed? Printer Installed 2010624-001 Korean 100 2010625-001 Korean 400 2010626-001 Korean 300 2010627-001 Korean 200 2010628-001 Korean 100 2010629-001 Finnish 400 Printer Installed 2010630-001 Finnish 300 Printer Installed 2010631-001 Finnish 200 Printer Installed 2010632-001 Finnish 100 Printer Installed 2010633-001 Finnish 400 2010634-001 Finnish 300 2010635-001 Finnish 200 2010636-001 Finnish 100 2010638-001 Portuguese 400 Printer Installed 2010639-001 Portuguese 300 Printer Installed 2010640-001 Portuguese 200 Printer Installed 2010641-001 Portuguese 100 Printer Installed 2010642-001 Portuguese 400 2010643-001 Portuguese 300 2010644-001 Portuguese 200 2010645-001 Portuguese 100 2010646-001 Russian 400 Printer Installed 2010647-001 Russian 300 Printer Installed 2010648-001 Russian 200 Printer Installed 2010649-001 Russian 100 Printer Installed 2010650-001 Russian 400 2010651-001 Russian 300 2010652-001 Russian 200 2010653-001 Russian 100 Revision B Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 4-33 Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies P/N (-001) Language Model Is Printer Installed? 2010656-001 Hungarian 400 Printer Installed 2010657-001 Hungarian 300 Printer Installed 2010658-001 Hungarian 200 Printer Installed 2010659-001 Hungarian 100 Printer Installed 2010660-001 Hungarian 400 2010661-001 Hungarian 300 2010662-001 Hungarian 200 2010663-001 Hungarian 100 2010664-001 Czech 400 Printer Installed 2010665-001 Czech 300 Printer Installed 2010666-001 Czech 200 Printer Installed 2010667-001 Czech 100 Printer Installed 2010668-001 Czech 400 2010669-001 Czech 300 2010670-001 Czech 200 2010671-001 Czech 100 2010672-001 Slovak 400 Printer Installed 2010673-001 Slovak 300 Printer Installed 2010674-001 Slovak 200 Printer Installed 2010675-001 Slovak 100 Printer Installed 2010676-001 Slovak 400 2010677-001 Slovak 300 2010678-001 Slovak 200 2010679-001 Slovak 100 2010680-001 Polish 400 Printer Installed 2010681-001 Polish 300 Printer Installed 2010682-001 Polish 200 Printer Installed 2010683-001 Polish 100 Printer Installed 2010684-001 Polish 400 4-34 Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 Revision B Calibration & Maintenance: Appendix D: Replacement Parts and Assemblies P/N (-001) Language Model Is Printer Installed? 2010685-001 Polish 300 2010686-001 Polish 200 2010687-001 Polish 100 2010688-001 Greek 400 Printer Installed 2010689-001 Greek 300 Printer Installed 2010690-001 Greek 200 Printer Installed 2010691-001 Greek 100 Printer Installed 2010692-001 Greek 400 2010693-001 Greek 300 2010694-001 Greek 200 2010695-001 Greek 100 Revision B Method of NIBP Determination (Auscultatory is labeled on left keypad) ProCare Patient Monitor Service Manual 2009381-001 4-35 A Revision B Appendix E – Electromagnetic Compatibility ProCare Patient Monitor Service Manual 2009381-001 4-37 4-38 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ProCare Monitor Electromagnetic Compatibility (EMC): ProCare Monitor Changes or modifications to this system not expressly approved by GE Medical Systems can cause EMC issues with this or other equipment. This system is designed and tested to comply with applicable regulation regarding EMC and must be installed and put into service according to the EMC information stated in this appendix. WARNING Use of portable phones or other radio frequency (RF) emitting equipment near the system may cause unexpected or adverse operation. WARNING The equipment or system should not be used adjacent to, or stacked with, other equipment. If adjacent or stacked use is necessary, the equipment or system should be tested to verify normal operation in the configuration in which it is being used. Guidance and Manufacturer’s Declaration – Electromagnetic Emissions The DINAMAP® ProCare Monitor is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the DINAMAP® ProCare Monitor is used in such an environment. Emissions Test Compliance Electromagnetic Environment – Guidance RF Emissions EN 55011 Group 1 The equipment uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF Emissions EN 55011 Class B Harmonic Emissions EN 61000-3-2 Class A Voltage Fluctuations/ Flicker Emissions EN 61000-3-3 Complies Revision B The equipment is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. ProCare Patient Monitor Service Manual 2009381-001 4-39 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ProCare Monitor Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The DINAMAP® ProCare Monitor is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the DINAMAP® ProCare Monitor is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Electrostatic Discharge (ESD) EN 61000-4-2 ± 6 kV contact ± 6 kV contact ± 8 kV air ± 8 kV air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical Fast Transient/Burst EN 61000-4-4 ± 2 kV for power supply lines ± 2 kV for power supply lines Mains power should be that of a typical commercial or hospital environment. ±1 kV for input/output lines ±1 kV for input/output lines Surge EN 61000-4-5 ± 1 kV differential mode ± 1 kV differential mode ± 2 kV common mode ± 2 kV common mode <5% Ut (>95% dip in Ut) for 0.5 cycles <5% Ut (>95% dip in Ut) for 0.5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <70% Ut (>30% dip in Ut) for 25 cycles <70% Ut (>30% dip in Ut) for 25 cycles <5% Ut (>95% dip in Ut) for 5 s <5% Ut (>95% dip in Ut) for 5 s 3 A/m 3 A/m Voltage dips, short interruptions and voltage variations on power supply input lines EN 61000-4-11 Power Frequency (50/60 Hz) Magnetic Field EN 61000-4-8 Mains power should be that of a typical commercial or hospital environment. Mains power should be that of a typical commercial or hospital environment. If the user of the equipment requires continued operation during power mains interruptions, it is recommended that the equipment be powered from an uninterruptible power supply or a battery. Power frequency magnetic fields should be at levels characteristics of a typical location in a typical commercial or hospital environment. NOTE: Ut is the AC mains voltage prior to application of the test level. 4-40 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ProCare Monitor Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The DINAMAP® ProCare Monitor is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the DINAMAP® ProCare Monitor is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Portable and mobile RF communications equipment should not be used closer to any part of the equipment, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF EN 61000-4-6 3 Vrms 150 KHz to 80 MHz 3 V rms Radiated RF EN 61000-4-3 3 V/m 80 MHz to 2.5 GHz 3 V/m d = 1.2 P d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer, and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site surveya, should be less than the compliance level in each frequency rangeb. Interference may occur in the vicinity of equipment marked with the following symbol: Note 1: At 80 MHz and 800 MHz, the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by reflection from structures, objects, and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the equipment is used exceeds the applicable RF compliance level above, the equipment should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the equipment. bOver the frequency range 150 KHz to 80 MHz, field strengths should be less than 3 V/m. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-41 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ProCare Monitor Recommended Separation Distances The table below provides the recommended separation distances (in meters) between portable and mobile RF communications equipment and the DINAMAP® ProCare Monitor. The DINAMAP® ProCare Monitor is intended for use in the electromagnetic environment on which radiated RF disturbances are controlled. The customer or the user of the DINAMAP® ProCare Monitor can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the DINAMAP® ProCare Monitor as recommended below, according to the maximum output power of the communications equipment. Separation Distance in Meters (m) According to Frequency of Transmitter Rated Maximum Output Power of Transmitter in Watts 150 kHz to 80 MHz a 0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 a d = 1.2 P 80 MHz to 800 MHz a d = 1.2 P 800 MHz to 2.5 GHz a d = 2.3 P At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. For transmitters rated at a maximum output power not listed above, the recommended separation distance [d] in meters (m) can be estimated using the equitation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE: These guidelines may not apply in all instances. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 4-42 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ProCare Monitor Compliant Cables and Accessories WARNING The use of accessories, transducers and cables other than those specified may result in increased emissions or decreased immunity performance of the equipment or system. The table below lists cables, transducers, and other applicable accessories with which GE Medical Systems claims EMC compliance. NOTE: Any supplied accessories that do not affect EMC compliance are not included. Part No Description Maximum Lengths 2008774-001 Turbo-Temp Oral Probe, Blue 3.0 m / 10 ft 2008775-001 Turbo-Temp Rectal Probe, Red 3.6 m / 12 ft 2016998-001 Dual Temp Cable 20 cm / 8 in 407705-006 Nellcor DuraSensor Reusable Finger Probe (DS100A) 0.9 m/ 3 ft 2008773-001 Nellcor Interface Cable, OxiSmart, DOC 10 Cable 3.3 m / 11 ft 2009743-001 Masimo PC08 Cable 2.5 m / 8.2 ft 2009745-001 Masimo Finger Sensor, Adult, Reusable N/A 316579-001 AC Cable, Hospital Grade, AHA 3.6 m / 12 ft 2013057-001 Universal AC/DC Adapter N/A ILC-1926 Isolated Level Converter N/A Temperature Cables and Probes Pulse Oximetry Cables and Sensors Accessories Revision B ProCare Patient Monitor Service Manual 2009381-001 4-43 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1926 Electromagnetic Compatibility (EMC): ILC-1926 Changes or modifications to this system not expressly approved by GE Medical Systems can cause EMC issues with this or other equipment. This system is designed and tested to comply with applicable regulation regarding EMC and must be installed and put into service according to the EMC information stated in this appendix. WARNING Use of portable phones or other radio frequency (RF) emitting equipment near the system may cause unexpected or adverse operation. WARNING The equipment or system should not be used adjacent to, or stacked with, other equipment. If adjacent or stacked use is necessary, the equipment or system should be tested to verify normal operation in the configuration in which it is being used. Guidance and Manufacturer’s Declaration – Electromagnetic Emissions The ILC-1926 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1926 is used in such an environment. Emissions Test Compliance Electromagnetic Environment – Guidance RF Emissions EN 55011 Group 1 The equipment uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF Emissions EN 55011 Class B Harmonic Emissions EN 61000-3-2 Not Applicable Voltage Fluctuations/ Flicker Emissions EN 61000-3-3 Complies 4-44 The equipment is suitable for use in all establishments, including domestic establishments and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1926 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ILC-1926 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1926 is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Electrostatic Discharge (ESD) EN 61000-4-2 ± 6 kV contact ± 6 kV contact ± 8 kV air ± 8 kV air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical Fast Transient/Burst EN 61000-4-4 ± 2 kV for power supply lines No AC Input No AC Input Surge EN 61000-4-5 ± 1 kV differential mode No AC Input Mains power should be that of a typical commercial or hospital environment. Voltage dips, short interruptions and voltage variations on power supply input lines EN 61000-4-11 Power Frequency (50/60 Hz) Magnetic Field EN 61000-4-8 ±1 kV for input/output lines ± 2 kV common mode <5% Ut (>95% dip in Ut) for 0.5 cycles <5% Ut (>95% dip in Ut) for 0.5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <70% Ut (>30% dip in Ut) for 25 cycles <70% Ut (>30% dip in Ut) for 25 cycles <5% Ut (>95% dip in Ut) for 5 s <5% Ut (>95% dip in Ut) for 5 s 3 A/m 3 A/m Mains power should be that of a typical commercial or hospital environment. If the user of the DINAMAP ILC-1926 requires continued operation during power mains interruptions, it is recommended that the Host monitor be powered from an uninterruptible power supply or a battery. Power frequency magnetic fields should be at levels characteristics of a typical location in a typical commercial or hospital environment. NOTE: Ut is the AC mains voltage prior to application of the test level. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-45 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1926 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ILC-1926 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1926 is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Portable and mobile RF communications equipment should not be used closer to any part of the equipment, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF EN 61000-4-6 3 Vrms 150 KHz to 80 MHz 3 V rms Radiated RF EN 61000-4-3 3 V/m 80 MHz to 2.5 GHz 3 V/m d = 1.2 P d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer, and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site surveya, should be less than the compliance level in each frequency rangeb. Interference may occur in the vicinity of equipment marked with the following symbol: Note 1: At 80 MHz and 800 MHz, the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by reflection from structures, objects, and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the equipment is used exceeds the applicable RF compliance level above, the equipment should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the equipment. bOver the frequency range 150 KHz to 80 MHz, field strengths should be less than 3 V/m. 4-46 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1926 Recommended Separation Distances The table below provides the recommended separation distances (in meters) between portable and mobile RF communications equipment and the ILC-1926. The ILC-1926 is intended for use in the electromagnetic environment on which radiated RF disturbances are controlled. The customer or the user of the ILC-1926 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ILC-1926 as recommended below, according to the maximum output power of the communications equipment. Separation Distance in Meters (m) According to Frequency of Transmitter Rated Maximum Output Power of Transmitter in Watts 150 kHz to 80 MHz a 0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 a d = 1.2 P 80 MHz to 800 MHz a d = 1.2 P 800 MHz to 2.5 GHz a d = 2.3 P At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. For transmitters rated at a maximum output power not listed above, the recommended separation distance [d] in meters (m) can be estimated using the equitation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE: These guidelines may not apply in all instances. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-47 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1926 Compliant Cables and Accessories WARNING The use of accessories, transducers and cables other than those specified may result in increased emissions or decreased immunity performance of the equipment or system. The table below lists cables, transducers, and other applicable accessories with which GE Medical Systems claims EMC compliance. NOTE: Any supplied accessories that do not affect EMC compliance are not included. Part No Description Maximum Lengths N/A RJ45 series Category 5 cable N/A 683235 Adapter Cable, RJ45 to DB15. 600 mm / 2 ft 683236 Adapter Cable, RJ45 to DB25 460 mm / 18 in 683242 Adapter Cable, RJ45 to DB9. 3.0 m / 10 ft Accessories 4-48 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 Electromagnetic Compatibility (EMC): ILC-1931 Changes or modifications to this system not expressly approved by GE Medical Systems can cause EMC issues with this or other equipment. This system is designed and tested to comply with applicable regulation regarding EMC and must be installed and put into service according to the EMC information stated in this appendix. WARNING Use of portable phones or other radio frequency (RF) emitting equipment near the system may cause unexpected or adverse operation. WARNING The equipment or system should not be used adjacent to, or stacked with, other equipment. If adjacent or stacked use is necessary, the equipment or system should be tested to verify normal operation in the configuration in which it is being used. Guidance and Manufacturer’s Declaration – Electromagnetic Emissions The ILC-1931 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1931 is used in such an environment. Emissions Test Compliance Electromagnetic Environment – Guidance RF Emissions EN 55011 Group 1 The equipment uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF Emissions EN 55011 Class A Harmonic Emissions EN 61000-3-2 Not Applicable Voltage Fluctuations/ Flicker Emissions EN 61000-3-3 Not Applicable Revision B The equipment is suitable for use in all establishments other than domestic and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes. ProCare Patient Monitor Service Manual 2009381-001 4-49 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ILC-1931 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1931 is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Electrostatic Discharge (ESD) EN 61000-4-2 ± 6 kV contact ± 6 kV contact ± 8 kV air ± 8 kV air Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. Electrical Fast Transient/Burst EN 61000-4-4 ± 2 kV for power supply lines N/A No AC Input Surge EN 61000-4-5 ± 1 kV differential mode N/A No AC Input Voltage dips, short interruptions and voltage variations on power supply input lines EN 61000-4-11 Power Frequency (50/60 Hz) Magnetic Field EN 61000-4-8 ±1 kV for input/output lines ± 2 kV common mode <5% Ut (>95% dip in Ut) for 0.5 cycles <5% Ut (>95% dip in Ut) for 0.5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <40% Ut (>60% dip in Ut) for 5 cycles <70% Ut (>30% dip in Ut) for 25 cycles <70% Ut (>30% dip in Ut) for 25 cycles <5% Ut (>95% dip in Ut) for 5 s <5% Ut (>95% dip in Ut) for 5 s 3 A/m 3 A/m Mains power should be that of a typical commercial or hospital environment. If the user of the ILC-1931 requires continued operation during power mains interruptions, it is recommended that the Host monitor be powered from an uninterruptible power supply or a battery. Power frequency magnetic fields should be at levels characteristics of a typical location in a typical commercial or hospital environment. NOTE: Ut is the AC mains voltage prior to application of the test level. 4-50 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity The ILC-1931 is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to assure that the ILC-1931 is used in such an environment. Immunity Test EN 60601 Test Level Compliance Level Electromagnetic Environment – Guidance Portable and mobile RF communications equipment should not be used closer to any part of the equipment, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distance Conducted RF EN 61000-4-6 3 Vrms 150 KHz to 80 MHz 3 V rms Radiated RF EN 61000-4-3 3 V/m 80 MHz to 2.5 GHz 3 V/m d = 1.2 P d = 1.2 P 80 MHz to 800 MHz d = 2.3 P 800 MHz to 2.5 GHz where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer, and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site surveya, should be less than the compliance level in each frequency rangeb. Interference may occur in the vicinity of equipment marked with the following symbol: Note 1: At 80 MHz and 800 MHz, the higher frequency range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by reflection from structures, objects, and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radio, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the equipment is used exceeds the applicable RF compliance level above, the equipment should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or relocating the equipment. bOver the frequency range 150 KHz to 80 MHz, field strengths should be less than 3 V/m. Revision B ProCare Patient Monitor Service Manual 2009381-001 4-51 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 Recommended Separation Distances The table below provides the recommended separation distances (in meters) between portable and mobile RF communications equipment and the ILC-1931. The ILC-1931 is intended for use in the electromagnetic environment on which radiated RF disturbances are controlled. The customer or the user of the ILC-1931 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ILC-1931 as recommended below, according to the maximum output power of the communications equipment. Separation Distance in Meters (m) According to Frequency of Transmitter Rated Maximum Output Power of Transmitter in Watts 150 kHz to 80 MHz a 0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 a d = 1.2 P 80 MHz to 800 MHz a d = 1.2 P 800 MHz to 2.5 GHz a d = 2.3 P At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. For transmitters rated at a maximum output power not listed above, the recommended separation distance [d] in meters (m) can be estimated using the equitation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE: These guidelines may not apply in all instances. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. 4-52 ProCare Patient Monitor Service Manual 2009381-001 Revision B Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 Compliant Cables and Accessories WARNING The use of accessories, transducers and cables other than those specified may result in increased emissions or decreased immunity performance of the equipment or system. The table below lists cables, transducers, and other applicable accessories with which GE Medical Systems claims EMC compliance. NOTE: Any supplied accessories that do not affect EMC compliance are not included. Part No Description Maximum Lengths 683235 Adapter Cable, RJ45 to DB15 600 mm / 2 ft 418497-002 Apex Pro Cable 1.8m / 6 ft Accessories Revision B ProCare Patient Monitor Service Manual 2009381-001 4-53 Appendix E – Electromagnetic Compatibility: Electromagnetic Compatibility (EMC): ILC-1931 4-54 ProCare Patient Monitor Service Manual 2009381-001 Revision B Assembly Drawings & ProCare Schematics: Appendix D: Replacement Parts and Assemblies 5 Assembly Drawings & ProCare Schematics ProCare Patient Monitor Service Manual 2009381-001 Revision B or see Appendix C in Section 4 for Display Cover removal instructions. or Exploded Assembly Drawing ProCare Monitor (1 of 2) page 5-1/2 or PNEUMATIC TUBING ROUTING Exploded Assembly Drawing ProCare Monitor (front case) page 5-3/4 MAIN PROCESSOR P3V3 P5A C47 C50 C45 100nF 25V C48 25V 100nF 100nF 25V C44 C46 P3V3 P3V3 100nF 25V 100nF 25V R1 R117 100K 100K 100nF 25V 10K0 P3V3 R108 1K00 T_DE T_CLK T_TDI T_TDO T_TMS T_TRST 75R0 R201 TP26 75R0 99 92 93 94 95 98 C32 10P 0R0 C33 10P R90 RESET_PRI-0 P3V3 R120 R62 10M0 XL2 8.0MHZ 1K00 R115100K 100K ADC_PWM CS_DISPLAY PWM_VOL PWM_PITCH 59 70 82 81 52 51 85 88 64 44 P5D C12 10N0 C49 100NF 25V P5D C1 10U0 P3V3 C14 10N0 C52 100NF 25V C15 10N0 P5A C2 10U0 P3V3 C53 100NF 25V C3 10U0 C16 10N0 VDD5 VDD4 VDD3 PB0 PB1 PB2 PB3 PB4 PB5 PB6 PB7 DE TCLK TDI TDO TMS TRST 90 CLKOUT 87 EXTAL 86 XTAL 83 RESET 84 RSTOUT 43 ICOC10 42 ICOC11 41 ICOC12 40 ICOC13 39 ICOC20 38 ICOC21 37 ICOC22 36 ICOC23 R63 1K00 C54 P3V3SEC 100NF 25V VPP VDDH VDDA VSSA VDDF VSSF VDDSYN VSSSYN VSTBY TEST R95 1K00 R104 1K00 49 50 53 56 57 58 60 61 PUMP_ON DUMP_ON LATCHED_OVP DUMP2_ON ADULT-0 PNEU_RESET R116 TP34 TP33 100K 7 PA0 6 PA1 5 PA2 4 PA3 3 PA4 2 PA5 1 PA6 100 PA7 63 MISO 62 MOSI 65 SCK 66 SS R206 Place near U9 R103 RESET_TEST-0 U9 MMC2107 PC0 PC1 PC2 PC3 PC4 PC5 PC6 PC7 PD0 PD1 PD2 PD3 PD4 PD5 PD6 PD7 PR_DI PR_CLK PR_ST1 PR_ST2 PR_ST3 PR_ST4 PR_ST5 PR_ST6 17 16 15 14 11 10 9 8 12 21 54 89 96 PR_VHON PR_M4 PR_M2 PR_M3 PR_M1 PR_EOP_EN PR_LAT PR_EOP 1K00 27 26 25 24 23 20 19 18 R114 SHDN_TO_PRI 1K00 SW_MUX RTC_DATA R113 TP42 RTC_CLK SPO2_RST-0 IPS_ON CS_SEC-0 R112 1K00 35 34 33 32 31 30 29 28 FSP_FROM_PRI FAST_OVP-0 DUMP_ON_MON D_FROM_TEMP D_TO_TEMP REM_ALARM-0 TEMP_PROBE_IN DUMP2_ON_MON R107 69 PE5 68 PE6 67 PE7 VSS5 R106 TP39 TP40 TP41 97 INT0 INT1 INT2 INT3 INT4 INT5 INT6 INT7 VSS4 SPI_MISO SPI_MOSI SPI_CLK 91 80 VRH 79 VRL 48 RXD1 46 RXD2 47 TXD1 45 TXD2 1K00 R119 D_FROM_COMM D_FROM_SPO2 D_TO_COMM D_TO_SPO2 55 VSS3 1K00 PQA0 PQA1 PQA3 PQA4 PQB0 PQB1 PQB2 PQB3 VSS2 V_DC_MON PUMP_I PT1 PT2 FPT V_BAT_MON_PRI VSS1 I_CHG 78 77 76 75 74 73 72 71 22 VDD2 R84 VDD1 13 PR_TH R98 P3V3 10K0 FSP_TO_PRI 1K00 TP30 R118 100K R190 10K0 PE7 needs to be high at start-up C5 100NF 25V SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (1 of 10) page 5-5/6 +3.3V SECONDARY POWER PBAT P3V3SEC C141 100nF 25V 16 VCC C115 R137 10K0 R141 49K9 P3V3SEC RESET U27 9 OSCOUT2 100nF 25V 10 OSCOUT1 11 OSCIN 12 RESET 8 GND Q14 Q13 Q12 Q10 Q9 Q8 Q7 Q6 Q5 Q4 3 2 1 15 13 14 6 4 5 7 1 VIN C106 2U2 C13 10N0 16V Q14 DTC143ZKA Logic U22 3 ON/OFFL LP2985 4 BYPASS VOUT GND 2 C56 SECONDARY PROCESSOR P3V3SEC R174 10K2 P3V3SEC 2 3 4 5 6 TP1 C35 C34 22P0 C103 12P0 XL3 32.768KHZ 1 OSCI 8 VDD R3 4K99 CLKOUT 7 2 OSCO SCL 6 RTC_CLK 3 INT SDA 5 RTC_DATA VSS 4 20 VDD RA0/AN0 RA1/AN1 RA2/AN2 RA3/AN3/VREF RA4 R175 10K2 9 OSC1 XL1 4.0MHZ R37 1K00 R2 4K99 100nF 25V 1 MCLR 22P0 U21 PCF8563T P3V3SEC C55 R43 1K00 R176 20K0 FSP_TO_PRI SEC_ALARM P3V3 25V 100nF PT2_SEC R177 20K0 REAL TIME CLOCK C105 10U0 P3V3SEC 74LV4060 V_BAT_MON_SEC I_CHG P5A2 5 R35 0R0 10 RB7 RB6 RB5 RB4 RB3 RB2 RB1 RB0/INT0 28 27 26 25 24 23 22 21 FAST_CHG LATCHED_OVP FAST_OVP-0 PNEU_OVERRIDE-0 RESET_PRI-0 SW_PWR-0 U3 PIC16LC73B OSC2 RC0 11 12 RC1/CCP2 13 RC2/CCP1 RC7/RX RC6/TX RC5/SDO RC4/SDI RC3/SCK RA5/AN4/SS AGND 8 18 17 16 15 14 7 VSS 19 FSP_FROM_PRI PNEU_RESET ADULT-0 SPI_MOSI SPI_CLK CS_SEC-0 SHDN_TO_PRI PS_ON SPI_MISO SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (2 of 10) page 5-7/8 P6VMIN C66 P5A 100nF 25V 1 VIN Pump Connector P5D PBAT P5D C39 J7 1 2 3 4 5 6 220U0 PBAT PUMP P5D DUMP P5D DUMP2 PBAT 3 1 3 ON/OFF U7 VOUT 5 C37 2U2 GND 2 16V D9 BAS16 C28 33U0 1 25V 3 25V 3 1 TP38 TP37 C65 100nF 25V P5D C140 33U0 PNEUMATICS LP2980-5V PBACK_IN PBACK_OUT XFPM-050 PT1 5V1 D1 3 VCC VOUT GND CAP 3 D10 BAS16 1 2 1 R180 6 C20 R36 R51 0R0 2K0 PT1 C67 100NF 25V 1K00 680P D27 BAS16 Spare Parts DUMP_ON_MON R202 3 100K Q7 5 SI2306DS DUMP_ON 3 9 2 DUMP2_ON_MON R203 11 Q24 SI2306DS DUMP2_ON 3 13 1 R204 100K R205 100K R173 100K U14 HCT04 P5A 4 R9 10K0 6 U14 HCT04 C60 100nF 25V U14 HCT04 U14 HCT04 3 R10 8 2 10K0 U13 8 FPT 1 4 10 R6 10K0 C17 10N0 12 R83 649K C64 2 R121 100K 10N0 Q17 PNEU_OVERRIDE-0 P5A TLC2272 1 100K U14 HCT04 R29 4K99 SI2306DS 3 P6VMIN 1 2 Q9 SI9410DY PUMP_ON 6 100nF 25V 7 2 1 VIN 3 Q8 SI9410DY FAST_OVP-0 4 6 7 R100 10K0 U6 GND 1 ADC_PWM U14 7 GND 2 VOUT 5 C36 2U2 16V R44 1K00 3 R99 10K0 4K99 HCT04 8 2 R5 10K0 2 LP2980-5V 3 ON/OFF 5 100nF 25V C43 470N P5A2 8 4 C61 VCC 14 R28 C58 5 P5A R75 0R10 C63 C59 100NF 25V 100nF 25V XFPM-050 PT2 VCC PUMP_I 3 GND 2 1 CAP 6 VOUT PT2 R50 R181 C21 1K00 680P 2K0 C57 100NF 25V PT2_SEC SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (3 of 10) page 5-9/10 BATTERY CHARGER DC Input Power V_IN J3 1 2 3 C104 100nF FS1 L4 2A5 900NH_0A7 2 D5 PBAT MIC4681-ADJ 2 VIN 1 BYG22 1 R96 301K 2 D8 C69 C29 33U0 SM6T39CA 1 SHDN 100nF 5 25V 1 SW 3 U17 FB 4 GND 6 7 8 1 2 D4 BYS10 68U0 L1 2 2 D3 1 BYG22 C70 C40 220U0 100NF 25V 25V R25 49K9 V_DC_MON C90 R87 100K 100nF BC_5V 8 MC78L05 IN R149 0R10 U2 OUT GND 2 3 6 7 R39 301K PBAT 1 C68 VR1 50K 100NF 25V CW VF = 6.9 V VC = 7.2 V R150 0R10 R42 10K0 BC_5V Battery Connector PBAT J2 1 C71 R13 10K0 4A0 FS4 100nF 25V 3 MBR2030CTL 3 D14 25V 1 C98 33U0 2 2 2 R11 R138 5 10K0 R92 100K R147 100K R76 0R10 10K0 OPA2340 U15 V = 0.191 V @ 75mA R182 0R10 8 OPA2340 1 4 R72 4K99 1 D12 3 BAS16 R81 200K C116 7 6 U15 100nF 25V R148 150K R61 R93 49K9 C91 100NF 25V R146 100K 249K C117 C118 100NF 25V 100nF 25V Q15 DTC143ZKA Logic R64 4K99 V_BAT_MON_SEC V_BAT_MON_PRI I_CHG FAST_CHG SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (4 of 10) page 5-11/12 +3.3 V SUPPLY PBAT P3V3 MIC4680-3V3 2 VIN C30 33U0 R101 10K0 25V U12 1 SHDN C74 100NF 25V GND 6 7 5 1 SW 3 Q12 C41 220U0 C75 100NF 25V 25V D6 BYG22 ANALOG VOLTAGE SUPPLY (+6.5 V) SI2307DS Logic 2 8 1 Q18 DTC143ZKA L2 FB 4 2 PS_ON 68U0 2 1 1 3 5 VIN C31 33U0 25V C93 100NF 25V 68U0 MIC3172 U16 4 EN L3 2 P6VMIN VSW 7 1 BYG22 FB 3 GND COMP 8 6 2 SGND 1 D7 2 R27 12K1 R65 1K00 C96 220U0 25V C97 220U0 25V C76 100NF 25V R33 2K87 C25 1U0 16V PROGRAM CONNECTOR J1 1 PGM_PS_ON-0 2 3 4 +5.0 V SUPPLY 5 2 VIN C38 2U2 16V C72 100NF 25V P5D MIC39101-5V 1 EN 5 6 U5 GND VOUT 3 FLG 4 7 8 C73 100NF 25V C4 10U0 RESET_TEST-0 SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (5 of 10) page 5-13/14 P3V3 R123 R59 100K 100K R58 100K R57 100K R56 10K0 R55 100K R54 100K R122 100K 100nF 25V 20 19 VCC 2OE 2 1A0 4 1A1 6 1A2 8 1A3 17 2A0 15 2A1 13 2A2 11 2A3 PR_DI PR_ST6 PR_ST5 PR_ST4 PR_ST3 PR_ST2 PR_ST1 PR_CLK U11 1Y0 18 16 1Y1 14 1Y2 12 1Y3 3 2Y0 5 2Y1 7 2Y2 9 2Y3 HCT240 1OE 1 P5A R97 1M00 PR_EOP 7 P3V3 8 4 Q11 P5D R60 30K1 R91 20K0 TLC2272 GND 10 P5A P5A PR_EOP_EN PRINTER INTERFACE P5D C79 Printer Connector J9 5 1 2 6 3 4 R89 30K1 U13 5 6 7 DTA143XKA Logic 8 R73 9 10 100R PBAT 11 12 13 14 R14 10K0 15 Q5 16 SI2307DS R15 17 2 18 1 PBAT_PR 10K0 19 20 3 21 PR_VHON C6 33U0 Q3 DTC143ZKA 22 25V Logic 23 P5A 24 25 26 R26 15K0 27 PR_TH P5D R53 2K0 PR_LAT Q4 DTC143ZKA C77 Logic C78 P3V3 100nF 25V 25V 100nF 2 13 5 VCC VS2 VS1 3 IN1 OUT1 4 PR_M1 PR_M3 PR_M2 PR_M4 7 IN2 15 IN3 LB1831M U4 OUT2 6 OUT3 14 11 IN4 OUT4 12 17 19 FG7 FG5 18 20 FG6 FG8 FG1 FG2 FG3 FG4 1 8 9 16 SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (6 of 10) page 5-15/16 AUDIO ALARMS P5D R49 1R00 P5D PWM_VOL 3 C R82 10K0 C7 10U0 Q27 MMBT3904LT1 1 B 16V E 2 C26 1U0 R24 100K 16V 1 D18 R41 10N0 4K99 U10 LM386 3 6 1 2 C11 10N0 R111 10K0 PWM_PITCH C10 C87 R71 15K0 100NF 25V 4 8 7 5 C8 10U0 3 R94 49K9 C88 100NF 25V R46 P5D 1K00 P5D R179 2K0 3 1 Q26 Logic HOST COMM 1 P5D C85 Logic 3 D16 5V1 P5D Q28 DTC143ZKA Logic 100nF 25V 2 C1+ C83 100NF 25V 15 VCC 4 C15 C2+ C82 100NF 25V 12 16 FON FOFF U8 ICL3221 V- 7 6 C211 T1IN 9 P5D Comms Connector C84 GND 1 TTL_TX 2 J6 TTL_RX 3 100nF 25V 5V_FUSED 4 GND 5 REMOTE_ALARM-0 6 C81 RS-232_TX 7 RS-232_RX 8 100nF 25V T1OUT 13 External DB15 Connector Mapping R1IN 8 R1OUT 1 EN D_FROM_COMM V+ 3 FS2 DTC143ZKA DTC143ZKA D15 5V1 0A3 D_TO_COMM Q25 Speaker Connector 100NF 25V R124 10K0 10N0 R178 1K00 2 P5D C89 SEC_ALARM 1 220U0 R74 2R70 C86 16V BAS16 J4 C42 1 INVALID J6 0 1 2 3 4 5 6 7 8 GND 14 FS3 0A3 Q13 DTA143XKA Logic R110 10K0 Q6 NDT3055L 1 REM_ALARM-0 2 NAME GND TTL_TX TTL_RX 5V_FUSED GND REMOTE_ALARM-0 RS-232_TX RS-232_RX DB15 1 2 3 4 7 8 11 13 D2 SM6T15CA R102 10K0 SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (7 of 10) page 5-17/18 Test Connector P3V3 R19 10K0 R22 10K0 R18 10K0 C92 R17 10K0 100NF 25V T_TDI 1 T_TDO 3 T_CLK 5 P3V3 J10 2 P5D PBAT Display Connector R125 10K0 4 6 7 RESET_TEST-0 T_TMS 8 1 9 2 SW_MUX 10 11 T_DE 12 T_TRST 14 3 4 CS_DISPLAY SW_PWR-0 SPI_MOSI SPI_CLK 13 R21 1K00 J5 5 6 7 8 V_IN R133 R134 R135 499 499 499 9 CHARGING_LED 10 R126 100K R127 100K ISOLATED POWER SUPPLY D19 L12 C147 330P R200 150 2 MCB1608S102E 2 VIN 1 BYG22 C148 C122 10U0 1 EN 100NF 25V P5TEMP MIC39101-5V 5 6 U29 GND VOUT 3 C121 10U0 FLG 4 7 8 T PBAT SP D21 1 R185 4K99 C124 10U0 C126 10U0 C149 MIC5270-5.0 100NF 25V 2 GND 5 -VIN 2 U28-VOUT SP C129 33U0 C151 25V R153 75R0 12 IPS_ON 15 SD-0 SW 11 6 SYNC C127 1N0 PKG_TYPE=0603 VIN R152 3K01 FB 3 4 VC ROC 14 5 RREF RCC 13 R151 D20 7 2 2 L11 8 11 L13 MCB1608S102E 5 MMZ2012R102A DO NOT POPULATE P5VSP T1 6395-TO12 C128 680P N5VSP 4 BYG22 100NF 25V ISOLATION BARRIER 2 VIN 1 BYG22 C123 10U0 C150 100NF 25V MIC39101-5V 1 EN 5 6 U30 GND VOUT 3 FLG 4 C125 10U0 7 8 12 13K7 0.1% SP LT1425CS U31 9 G3 G1 1 16 G4 G2 8 7 SG PG 10 C101 C102 1N0 1N0 C145 C146 1N0 1N0 3K15V 3K15V 3K15V 3K15V ISOLATION BARRIER SCHEMATIC SP T PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (8 of 10) page 5-19/20 SpO2 Module Connector 10 pin 1 1 2 J8 8 1 7 3 6 4 5 2 6 7 8 9 6 7 GND 3 8 N/C 10 N/C 11 LED + 2 13 3 14 4 FL2 INNER SHIELD INNER SHIELD DET ANODE DET CATHODE LED - RED LED CATHODE RCAL 10 DIGICAL 11 12 1 13 Z>200/100MHZ NC 12 GND FL1 4 9 LED - IR LED CATHODE 5 5 DET - C MASIMO LED + 4 4 DIGICAL NELLCOR RCAL - RETURN 3 Z>200/100MHZ 3 GND TO J1 ON NELLCOR MP506 FL3 2 J11 DET + A SpO2 Patient Connector Allow 4mm clearance between these signal and all other board signals 14 15 ANALOG GND OUTER SHIELD DET CATHODE DET ANODE 16 2 1 Z>200/100MHZ J16 DET + A INNER SHIELD INNER SHIELD TO J1 ON MASIMO MS-5 1 2 DET - C N/C OUTER SHIELD IR LED CATH OUTER SHIELD RED LED CATH N/C P5VSP 3 4 5 6 NC 2 7 D35 8 9 10 BAV199 3 NC 1 P5VSP P3V3 Q19 P3V3 Logic R144 249 R183 10K0 U23 4N35 DTA143XKA 1 2 AN C B CTH E SP SpO2 Module Connector 14 pin 5 6 4 N5VSP P5VSP R129 10K0 SP C119 100nF 25V SPO2_RST-0 J12 P5VSP P5D 1 8 7 6 5 VCC AN VE VO GND CTH GND 4 2 3 C 3 Q16 MMBT3904LT1 1 B RESET 5 R142 3K01 GND 6 +12V (N/A) 7 ANALOG OUT (N/A) 8 TRANSMIT (MP506 OUT) 9 RECEIVE (MP506 IN) 10 2 E 11 R136 499 P5VSP P3V3 CTS (MP506 IN) Q20 Logic R145 249 2 3 AN CTH VCC VE SP VO GND SP J17 R139 1K00 1 2 3 7 4 SP 6 5 5 6 7 8 9 SP 10 ISOLATION BARRIER SCHEMATIC GND R184 1K00 100nF 25V 8 +5V DIGITAL 14 C120 U26 HCPL-2601 GND 13 SP DTA143XKA TO J2 ON NELLCOR MP506 +5 ANALOG 12 D_FROM_SPO2 D_TO_SPO2 GND 3 U25 HCPL-2601 NELLCOR C-LOCK 2 PWA, MAIN BOARD, PROCARE MASIMO +5V GND GND TO J3 ON MASIMO MS-5 GND RESET RECEIVE (MS-5 IN) TRANSMIT (MS-5 OUT) SP Schematic - Main Board P/N 2008855 Rev B (9 of 10) page 5-21/22 P5TEMP P5TEMP R166 1K00 U36 TL431 C137 R186 10K0 1 8 T C130 10U0 2 3 6 7 16V R172 Temperature Probe Connector 13K7 0.1% P5TEMP R193 10K0 3 L14 J14 2 3 5 R167 100R 3 DIN 20 DOUT 21 SCLK 22 AD0 AD1 AD2 AD3 3 4 X_IN X_OUT 5 RESET-0 DSYNC-0 POL BUFEN PDWN-0 6 5 4 3 DRDY_FROM_T_ADC 2 1 P5TEMP ADS1240 Temp PGM Connector 6 T DO NOT POPULATE J15 R156 6 18 24 7 10K0 R155 10K0 DGND1 2 DGND2 8 ISOLATION BARRIER T T C136 100NF 25V D23 D_TO_T_ADC D_FROM_T_ADC SCLK_TO_T_ADC V_THERM 1K00 C152 BAS16 1 11 12 13 14 16 AGND R157 BLM11B221SB 4 CLK_TADC BAS16 BLM11B221SB L9 1 DVDD 1 9 REF+ 10 REF- D24 1 17 AVDD DRDY-0 23 15 AINCOM U34 CS-0 19 T P5TEMP P5TEMP 100nF 25V 100NF 25V T P5TEMP C138 T T P5TEMP P5TEMP L7 1 1 R168 R197 R164 30R 499K R194 R195 R196 30R 30R 30R D28 BAS16 T Q21 SI2307DS 2 1 R192 10K0 3 3 R171 1K00 19 20 1 2 3 D29 BAS16 1 R160 MON_HTR 10K0 18 C134 T 100NF 25V P5TEMP 17 1 D22 22P0 BAS16 C131 BLM11B221SB XL4 4.0MHZ 14 13 12 11 10 9 RB7 RB6 RB5 RB4/PGM RA0/AN0 RB3 RB2/TX RA1/AN1 8 RA2/AN2/VREF RB1/RX 7 RA3/AN3/CMP1 RB0/INT RA4/T0CKI/CMP2 OSC1 U35 PIC16F628 OSC2/OUT C132 T 3 15 VDD1 16 VDD2 4 MCLR-0 R162 0R0 VSS1 5 VSS2 6 T T R191 2K21 R154 10K0 U33 4N35 T 100nF 25V BLM11B221SB L10 100nF 25V P5TEMP C133 T 3 P3V3 2 2K0 AN C B CTH E D_FROM_TEMP 5 6 4 Q23 D_TO_TEMP DTA143XKA Logic R198 10K0 R188 100K 5 6 4 T T R169 1K00 U32 4N35 T C AN B E CTH 1 2 R187 10K0 T 22P0 3 D25 TEMP_PROBE_IN Q22 BAS16 1 T SI2306DS 3 1 2 R158 10K0 T P3V3 C142 2U2 V_JUMPER C135 100NF 25V R165 499K R132 10K0 R189 EN_HTR 499K Temp Probe Switch Connector P5D J13 1 2 3 16V T T T SCHEMATIC PWA, MAIN BOARD, PROCARE Schematic - Main Board P/N 2008855 Rev B (10 of 10) page 5-23/24 P5D C3 P5D PDG_L[0:12] 1 23 Y10 Y11 EL Y12 E Y13 D1 P5D HCT132 Q1 DTC143ZKA L L Q3 DTC143ZKA R42 180R PDG_L12 100NF 50V DG12 SA0 2 SA1 15 SA2 13 SA3 11 A SA4 HCT132 C 14 P5D U1 HCT132 P5D U1 GND S1 R23 S2 330 SA1 330 R24 SA2 S3 R25 330 SA3 S4 330 R26 SA4 S5 R27 330 SA5 330 R28 SA6 S6 C4 SA0 2 SA1 15 SA2 13 SA3 11 8 2 IO1 CLK IO2 Q0 IO3 OE1 3 19 DS0 1 19 HCT299 U4 IO4 OE2 IO5 S0 IO6 S1 IO7 Q7 P5D CLR 9 DS7 GND 9 PS0 13 PS1 6 PS2 14 PS3 5 PS4 15 PS5 4 PS6 16 PS7 C5 100NF VCC 11 12 8 IO0 DS0 IO1 CLK IO2 Q0 IO3 HCT299 2 OE1 U3 3 OE2 1 S0 19 19 S1 IO4 IO5 IO6 IO7 Q7 P5D CLR 9 9 GND DS7 7 PS8 13 PS9 6 PS10 14 PS11 5 PS12 15 PS13 4 PS14 16 PS15 330 16 S10 330 R35 13 S11 R30 330 3 S12 330 R34 5 S13 R31 330 11 S14 330 R33 15 S15 R32 330 330 DG7 7 DP 1 DIG1 2 DIG2 6 DIG3 8 DIG4 DG10 15 Q9 DTC143ZKA L SA2 13 SA3 11 g D2 E1 e 9 5 c E2 SA4 d F DP G 7 SA5 3 SA6 14 10 DP 4 COM 12 12 COM 17 17 COM LED29 LTC2621JD A a B C D1 f b g D2 E1 S8 e E2 c d F DP 7 SA5 3 SA6 14 C DIASTOLIC LED18 DMR20A-1 RED a C f D1 b SA0 2 SA1 15 SA2 13 SA3 11 g D2 L Q10 DTC143ZKA L DG9 R50 180R DG8 R51 180R Q11 DTC143ZKA Q12 DTC143ZKA L L Q14 DTC143ZKA 620 12 620 R103 6 S11 R99 620 1 S12 620 R102 4 S13 R100 620 S14 620 R101 G DP COM COM COM F S0 a B/L2 a f D b L1 g E F e 7 G 3 DP DG0 2 DIG1 DG1 5 DIG2 DG2 138 DIG3 L1,L2,L3 f b b g g e c e c L2 d f c d DP 12 R105 200 11 200 R110 3 S3 R106 200 4 S4 200 R109 2 S5 R107 200 15 S6 200 R108 d DP R111 S2 S1 a L3 C/L3 16 LED28 LTC4624Y RED 200 DP YELLOW A/L1 a B/L2 C/L3 D f a b F e 8 G 6 DP DG6 1 DG1 DG7 5 DG2 DG8 7 DIG3 14 L1,L2,L3 f L1 g E E1 e E2 d F C DP G SA4 7 SA5 3 SA6 14 SA1 15 SA2 13 SA3 11 9 5 c d F DP G SA4 7 SA5 3 SA6 14 10 DP 4 COM 12 COM 17 COM e E2 12 COM 2 g E1 4 COM b D2 10 DP f D1 5 c a B SA0 17 COM LED27 LTC4624JD A C D1 f b g D2 E1 R97 12 S1 R37 330 11 S2 330 R96 3 S3 R57 330 4 S4 330 R95 2 S5 R58 330 15 S6 330 R94 8 S0 a B e E2 c d F b f e e c a b L3 f g e d DP b g c L2 d c d DP DP 330 DG9 DP G 1 5 DG10 DP DG11 COM 7 14 COM 6 C/L3 a D f a b f b g g e d DP 19 3 18 4 b g E F e f L1 17 5 16 6 15 R120 7 14 200 8 13 R122 9 12 200 10 11 R124 a L3 b f g e c b g e c c L2 G d DP DG1 d DP d DP DP DG2 DIG3 L1,L2,L3 COM a f c d 20 R119 200 200 R121 200 R123 200 S15 S14 S13 S12 S11 S15 S[0:15] AUTO/TIME b e a RED 2 A/L1 B/L2 GURX10D 1 RED LED11 LTC2623JD g c LED9 SPO2 R118 13 R112 620 15 S10 620 R117 2 S11 R113 620 6 S12 620 R116 5 S13 R114 620 16 S14 620 R115 S8 g E G R98 S10 PULSE A/L1 LED19 DMR20A-1 RED A 9 a f D 15 620 RED a B R104 S9 S9 R29 DG9 b 2 SA1 A S9 DG8 f D1 SA0 TEMP LED30 LTC4627JD 20 P5D SA4 17 14 C 4 B 17 R36 a B 10 A 12 S8 14 COM 4 18 3 SA6 COM 10 10 P5D R46 180R MAP DG4 12 Q8 DTC143ZKA L LED16 DMR20A-1 RED A 7 SA5 COM DG3 11 7 11 DP 5 20 IO0 13 SA3 SA4 DP G 9 100NF VCC SA2 LED17 DMR20A-1 RED P5D needs pull up on main board P5D Q7 DTC143ZKA L R47 180R Q26 2SB1188 SA[0:6] 330 R56 100R Q6 DTC143ZKA LED10 DMR20A-1 RED DG1 U1 SA0 15 5 c d F 17 S0 GND e E2 12 R14 2 SA1 9 DG0 100R E1 4 HCT132P5D R55 100R GND b SA0 g D2 10 100NF 1 J1 3 SA6 f D1 7 SA5 a B 9 P5D C9 SYSTOLIC LED13 DMR20A-1 RED Q13 DTC143ZKA L P5D R131 L R48 180R R2 10K0 1% Q2 2SB1188 V_IN 10 PBAT 9 SPI_CLK 8 SPI_MOSI 7 SW_PWR 6 CS_DISPLAY 5 P5D 4 SW_MUX 3 GND 2 Q5 DTC143ZKA L R49 180R R12 10K0 1% Q25 2SB1188 DG[0:12] 5 PBAT Q4 DTC143ZKA L R44 180R 12 C1 CONN_S_2X5_HDRF R45 180R R11 10K0 1% Q24 2SB1188 15 R39 200R R40 470K R43 180R R10 10K0 1% Q23 2SB1188 DG7 PDG_L12 R52 180R R9 10K0 1% Q22 2SB1188 DG6 PDG_L11 14 GND U1 BAT54C PDG_L10 19 R53 180R R8 10K0 1% Q21 2SB1188 16 Y15 GND 20 R41 180R R7 10K0 1% Q20 2SB1188 13 Y14 P5D R61 10K0 1% PDG_L9 R6 10K0 1% Q19 2SB1188 PDG_L9 HEF4514 U2 PDG_L8 17 R5 10K0 1% Q18 2SB1188 PDG_L8 18 18 R3 10K0 1% Q17 2SB1188 PDG_L7 R60 10K0 1% 17 10 9 Y9 PDG_L7 R4 10K0 1% Q16 2SB1188 PDG_L6 Y8 16 PDG_L6 4 R13 10K0 1% Q15 2SB1188 DG5 P5D 4 5 R1 10K0 1% DG6 DS7 GND Y7 PDG_L5 DG11 9 Y6 15 PDG_L4 PDG_L5 Q7 CLR Y5 6 DG4 IO7 A3 PDG_L3 7 PDG_L4 IO6 S1 Y4 DG3 S0 5 A2 PDG_L2 8 PDG_L3 IO5 22 Y3 PDG_L1 10 DG2 IO4 21 DIG_MUX3 A1 9 PDG_L2 HCT299 U5 OE2 DIG_MUX2 14 Y2 DG5 IO3 6 Y1 A0 DG1 Q0 3 PDG_L1 1 19 P5D IO2 OE1 3 19 CLK 2 DIG_MUX1 PDG_L0 2 P5D IO1 DIG_MUX0 13 DG10 12 8 DS0 7 PDG_L10 100R IO0 PDG_L0 DG11 20 VCC 11 11 PDG_L11 Y0 DG2 24 VCC DG0 C2 100NF R127 PBAT 100NF e c c d 620 d DP DP DG3 DP DG4 DG5 DG6 DG11 RED A/L1 a B/L2 C/L3 D f E F e 7 G 3 DP 1 DIG1 14 DIG2 11 DIG3 8 DIG4 12 L1,L2,L3 a b f g a b L1 f g c d e f g c d DP a L3 b e c L2 d DP b g e c d DP DP 17 18 10 PS[0:15] Q36 DTD123EK L SCHEMATIC L Q38 DTD123EK L Q39 DTD123EK L Q40 DTD123EK L S15 S14 S13 S12 S11 Q37 DTD123EK Q41 DTD123EK L Q42 DTD123EK PS15 L PS14 Q35 DTD123EK PS13 L S10 S9 S8 Q34 DTD123EK S[0:15] PS12 L PS11 Q33 DTD123EK PS10 L S7 S6 Q32 DTD123EK PS9 L PS8 Q31 DTD123EK PS7 L S5 S4 Q30 DTD123EK PS6 L PS5 Q29 DTD123EK PS4 L S3 S2 Q28 DTD123EK PS3 Q27 DTD123EK PS1 PS0 L L PS2 S0 S1 DTC143ZKA is a digital transistor with a 4K7 base resistor and a 47K base pull-down resistor DTD123EK is a digital transistor with a 2K2 base resistor and a 2K2 base pull-down resistor PROCARE USER INTERFACE BOARD Schematic User Interface Board P/N 2008741 Rev B (1 of 2) page 5-25/26 PCB BOARD SWITCH LOCATIONS PBAT C12 C11 C10 100NF 100NF 100NF 50V 50V 50V C8 C6 C7 16V 16V 16V 47U0 SILENCE 2 5 INFLATE ALARM 1 3 CYCLE 7 0 HISTORY 6 8 PRINT 4 9 ON/OFF P5D 47U0 + - 47U0 MENU V_IN DG[0:12] DG3 LED37 APTD3216QYC GREEN PDG_L[0:11] DG4 CHARGING R126 10K0 1% LED21 APTD3216QYC GREEN DG5 R135 10K0 1% SW9 SW_PWR A B LED66 APTD3216QYC GREEN ON/OFF CHARGING degF Q43 DTD123EK L D2 BAT54C LED61 AP3216SURC RED R20 38R3 R18 38R3 S7 SW0 A LED62 AP3216SURC RED degC LED65 APTD3216QYC GREEN SW_MUX B HISTORY BATT LOW RIGHT BATT LOW LEFT LED56 APTD3216SYCK YELLOW LED54 AP3216SURC RED BELL LED69 APTD3216SYCK YELLOW LED60 APTD3216SYCK YELLOW R133 38R3 R75 22R S7 S15 R136 10K0 1% LED68 APTD3216QYC GREEN R77 22R R134 38R3 S15 S7 BATTERY MIDDLE BATTERY RIGHT LED55 AP3216SURC RED S15 2 1 LED15 APTD3216QYC GREEN BATTERY LEFT LED1 APTD3216QYC GREEN LED57 APTD3216QYC GREEN LED5 APTD3216QYC GREEN R16 22R R74 22R S15 S7 PDG_L0 SW1 A B ALARM PDG_L1 DG12 SW2 D6 A B SILENCE BAT54C B PULSE LOW minus R125 10K0 1% SW3 A LED67 AP3216SURC RED PDG_L2 R64 38R3 PDG_L3 CYCLE SW4 A D5 BAT54C B S8 PDG_L4 MENU LED53 AP3216SURC RED PULSE HIGH LED40 AP3216SURC RED LED52 AP3216SURC RED SpO2 LOW SpO2 HIGH LED50 AP3216SURC RED DIA LOW LED49 AP3216SURC RED DIA HIGH LED46 AP3216SURC RED LED44 AP3216SURC RED SYS LOW LED42 AP3216SURC RED SYS HIGH LED38 AP3216SURC RED LED39 AP3216SURC RED LED41 AP3216SURC RED LED51 AP3216SURC RED LED48 AP3216SURC RED LED47 AP3216SURC RED LED45 AP3216SURC RED LED43 AP3216SURC RED R79 22R R65 22R R63 22R R38 22R R21 22R R19 22R R17 22R R15 22R S6 S7 S5 S4 S3 S2 S1 S0 SW5 A B PDG_L5 INFLATE DG0 DG1 SW6 D4 A SW7 A DG12 PDG_L6 MINUS BAT54C DG2 B B PDG_L7 AL VOL RIGHT LED2 APTD3216QYC GREEN AL VOL LEFT LED58 APTD3216QYC GREEN LED8 APTD3216QYC GREEN LED6 APTD3216QYC GREEN LED59 APTD3216QYC GREEN R70 22R R72 22R R22 22R LED3 APTD3216QYC GREEN AL VOL MIDDLE PUL VOL LEFT LED64 APTD3216QYC GREEN PUL VOL MIDDLE LED7 APTD3216QYC GREEN PLUS SW8 A D3 BAT54C LED63 APTD3216QYC GREEN B PRINT PUL VOL RIGHT LED35 APTD3216QYC GREEN LED22 APTD3216QYC GREEN LED34 APTD3216QYC GREEN AUTO RIGHT HISTORY LED20 APTD3216QYC GREEN LED4 APTD3216QYC GREEN R68 22R R80 22R LED32 APTD3216QYC GREEN AUTO LEFT LED26 APTD3216QYC GREEN INF PRES RIGHT LED36 APTD3216QYC GREEN LED70 APTD3216QYC GREEN LED14 APTD3216QYC GREEN LED33 APTD3216QYC GREEN R90 22R R137 38R3 R89 22R R87 22R 2 INF PRES MIDDLE LED31 APTD3216QYC GREEN 1 LED25 APTD3216QYC GREEN LED24 APTD3216QYC GREEN INF PRES LEFT LED12 APTD3216QYC GREEN LED23 APTD3216QYC GREEN PDG_L8 S7 S15 S15 R62 22R S7 S7 S15 S15 S14 PROCARE USER INTERFACE BOARD S14 S13 R85 22R S12 R84 22R S11 R82 22R S10 Schematic User Interface Board P/N 2008741 Rev B (2 of 2) page 5-27/28 0086 2009381-001 B gemedical.com World Headquarters GE Medical Systems Information Technologies, Inc. 8200 West Tower Avenue Milwaukee, WI 53223 USA Tel: + 1 414 355 5000 1 800 558 5120 (US only) Fax: + 1 414 355 3790 European Representative GE Medical Systems Information Technologies GmbH Munzinger Straße 3-5 D-79111 Freiburg Germany Tel: + 49 761 45 43 - 0 Fax: + 49 761 45 43 - 233 Asia Headquarters GE Medical Systems Information Technologies Asia; GE (China) Co., Ltd. 24th Floor, Shanghai MAXDO Center, 8 Xing Yi Road, Hong Qiao Development Zone Shanghai 200336, P.R. China Tel: + 86 21 5257 4650 Fax: + 86 21 5208 2008