Download Automatic injection device with torsional spring
Transcript
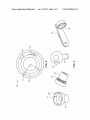
US 20130096513A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0096513 Al Smith (43) Pub. Date: (54) AUTOMATIC INJECTION DEVICE WITH (30) Apr. 18, 2013 Foreign Application Priority Data TORSIONAL SPRING Jul. 8, 2010 (75) Inventor: (EP) ................................ .. 10168927.1 Christopher James Smith, Cheshire (GB) Publication Classi?cation (73) Assignee: SANOFI-AVENTIS DEUTSCHLAND GMBH, Frankfurt am Main (DE) (21) Appl. No.: 13/579,081 (22) PCT Filed: Feb. 15, 2011 PCT N .: PCT/EP2011/052226 (51) Int- Cl A61M 5/315 (2006.01) (52) US. Cl. CPC ................................. .. A61M5/3155 (2013.01) 86 ( ) O USPC ........................................................ .. 604/211 (57) ABSTRACT § 371 (0X1), (2), (4) Date? Dec- 27, 2012 Related US‘ Application Data A semi-automatic “?xed” dose injection device is disclosed containing a reservoir of medicament, Where a trigger con Provisional application No. 61/305,436, ?led on Feb. 17, 2010. mined injection of medicament from the reservoir through a trols the unWmdmg of a tors1onal spring to cause a predeter (60) disposable needle. W1 Patent Application Publication Apr. 18, 2013 Sheet 1 0f 2 US 2013/0096513 A1 Patent Application Publication Apr. 18, 2013 Sheet 2 0f 2 US 2013/0096513 A1 Apr. 18, 2013 US 2013/0096513 A1 AUTOMATIC INJECTION DEVICE WITH TORSIONAL SPRING needle by the action of stored energy, preferably from a, e.g. Wound, dose spring, Within the device. The user, hoWever, may have to manually insert the needle before the injection, CROSS REFERENCE TO RELATED APPLICATIONS remove the needle after the injection, and/or activate the trigger in order to complete the injection. The user may also have to “recharge” the dose spring betWeen doses. This may [0001] The present application is a US. National Phase Application pursuant to 35 U.S.C. §371 of International Application No. PCT/EP2011/052226 ?led Feb. 15, 2011, Which claims priority to US. Provisional Patent Application No. 61/305,436 ?led on Feb. 17, 2010 and European Patent Application No. 10168927.1 ?led Jul. 8, 2010. The entire disclosure contents of these applications are herewith incor porated by reference into the present application. FIELD OF INVENTION [0002] This disclosure relates to a “?xed” dose injection device that may deliver a predetermined dose of medicament from a cartridge preferably through a single needle, particu larly semi-automatically, When a user activates a trigger. BACKGROUND be accomplished as part of the dose setting procedure. Energy may be stored in the dose spring during dose setting, Which energy may be released for injecting the dose. [0011] Using the “?xed” dose mechanism of this disclosure it is possible to con?gure the mechanism Where the ?xed dose is anyWhere betWeen 0.01 ml to approximately 0.30 ml (1 insulin unit to about 30 insulin units). Because the disclosed device delivers only a predetermined ?xed dose, the dose dial may have only markings for the single predetermined ?xed dose. No other dose markings are needed. The user may rotate the dose dial to the marking for the ?xed dose Whereupon the dose dial clicks as a ratchet on the drive shaft engages a gear tooth on the gear. At this point the dose dial expediently remains in the “set” position. The user may be prevented from dialing doses smaller than the ?xed dose by the action of the Multi-use and disposable injection devices are in dose spring, e. g. a torsion action. If the user does not rotate the common use for the delivery of injected medication. Such dose dial suf?ciently to engage the dose dial ratchet, then as devices are needed so that a user may take repeated doses of soon as the user releases the dose dial the dose spring Will rotate the dose dial back to the Zero unit position. The user [0003] a medication at speci?c time intervals, for example rapid acting or basal insulins, GLP-l, heparin, etc. Conducting repeated injections is both inconvenient and confusing to some users, especially those users With visual imparity or cognitive de?ciencies. [0004] Accordingly, them is a strong need to provide inj ec tion devices that are easy to use and that alloW a user to set a predetermined “?xed” dose of a medicament. [0005] It is an object of this disclosure to provide a novel injection device, preferably an improved injection device. [0006] This object may be achieved by the subject matter of the independent claims. Advantageous embodiments and re?nements are the subject matter of the dependent claims. [0007] This disclosure facilitates solving the above-de scribed problems, for example, by providing a, preferably semi-automatic, injection device Were the user can only dial predetermined doses of medicament. These and other advan tages Will become evident from the folloWing more detailed description of the invention. [0008] The claimed subject matter does have various advantages Which, inter alia, Will become apparent from the description beloW. [0012] The setting of the ?xed predetermined dose may be controlled by the interaction of the gear, the trigger, and the drive shaft, Which may be, preferably permanently, connected to the dose dial. During dose setting the gear may be pre vented from rotation by the trigger. The trigger may have a set of teeth on an inner surface that engages complementary teeth on the outer side of the gear. The drive shaft preferably has a ratchet arm that engages one or more limited number of teeth, Which are preferably equally spaced an angle 0t from each other. The teeth may be provided on the proximal side and/or on an inner surface of the gear. Preferably, this angle 0t is at least about 20 degrees, most preferably in the range of about 30 degrees to about 180 degrees. The minimum end of the preferable range may be determined by the smallest rotation that can be easily distinguished by the user so it is clear Whether or not a dose has been set. The maximum end of the preferable range may be determined by the greatest rotation that a user can comfortably achieve in one single movement Without signi?cantly altering their grip during dose setting. SUMMARY [0009] may be prevented from dialing larger doses by one or more mechanical stops limiting the travel of the dose dial. According to an exemplary arrangement the injec tion device of this disclosure, for example a ?xed dose inj ec tion device, is a multiple use device, meaning that a number of injections can be administered from the same medicament container or cartridge until that container is empty. The device may be made as a disposable variant Where the entire device is discarded once the medicament container is empty, or as a reusable variant, Where the user can discard and replace the empty medicament container and reuse the device mecha nism. For each separate inj ection the user may manually attach a neW sterile needle. After the injection this needle may be manually removed and discarded. [0010] The injection device of this disclosure may be a “semi-automatic” injection device. This means that the liquid medication Will be delivered automatically through the For example, a 120 degrees rotation may be more comfortable for a user, but 180 degrees is also possible. Furthermore, the angle 0t is preferably a divisor of 360 degrees so that the teeth may be evenly spaced around the inner side of the gear. In variable dose injectors, each tooth on the inner side of a gear usually corresponds to one index of the dose dial, for example, 0.01 ml (1 insulin unit) for a 21 unit con?guration or 0.02 ml (2 insulin units) for a 42 unit version. In these non ?xed dose devices, the angle betWeen the teeth is alWays less than 16 degrees. This is in contrast to this disclosure Where the angle 0t is preferably alWays greater than about 20 degrees. When dialing a dose in these prior art devices the user rotates the dose dial and the drive shaft ratchet clicks over the desired number of teeth. In this manner a number of different doses could be set each time the device is used. This is preferably not possible in the ?xed dose device of this disclosure, Which requires rotation to overcome only a single tooth to set the Apr. 18, 2013 US 2013/0096513 A1 same “?xed” predetermined dose each time the device is used. Preferably, a rotation Which may overcome more than one tooth is prevented. [0013] Rotating the dose dial may also store energy, e.g. torsional energy, Within the dose spring. When the trigger is activated, e.g. pressed, the trigger and the gear may disen gage. Particularly, the teeth on the gear, preferably outer teeth on the gear, may disengage from the corresponding teeth on the trigger and the gear becomes free to rotate. The dose spring may drive the drive shaft in rotation, Which in turn preferably rotates the gear, particularly, because the ratchet arm is engaged With teeth of the gear, preferably inner teeth of the gear. The gear may have one or more internal threads that are engaged With one or more external threads of the plunger. The pitch [3 of the respective thread, preferably of the threads of the plunger and/or of the gear, may be selected and matched so that rotation of the gear, Which is expediently constrained in the axial direction, Will cause the non-rotating plunger to move axially. Preferably, the angle 0t and the pitch [3 are selected so that the axial advance of the plunger is set to a particular value corresponding to the desired ?xed dose. The axial advance of the plunger may be determined by the cal culation: [0014] More speci?cally, angle 0t is preferably in the range from about 30 degrees to about 180 degrees. The pitch [3 of a thread on plunger and/or gear is preferably from about 1.5 mm to about 12 mm. Expressed differently, it is preferred that the ratio of ot/ [3 is in the range from about 10 to about 20 degrees/mm. [0015] Preferably the limited number of teeth on the gear that dictate or contribute to the “?xed” predetermined dose are evenly spaced around the circumference of the gear. The angle 0t betWeen these teeth preferably corresponds to the angle that the drive shaft Will be rotated When the ?xed dose is delivered. For example, if the gear contains four teeth then the angle 0t betWeen them Will be 90°. If the drive plunger set any dose beloW the “?xed dose” determined by the spacing of the gear teeth. The fact that the dose dial immediately rotates back to Zero units, rather than remaining at the marker for the ?xed dose, Will alert the user to the fact that the dose Was not properly set. This should prevent the user from attempting to deliver a Zero dose. This places an ergonomic limit on hoW far the user can be expected to rotate the dose dial in one movement. It is therefore preferred that there should be a minimum of tWo gear teeth. This Would mean that the maximum rotation the user Would have to apply Would be 1 80°. [0017] The maximum dose of the device may be limited by adding features that Will limit the rotation of the dose dial (and hence drive shaft). This rotation limit, Will be designed to ensure that the user Will alWays be able to dial the ratchet arm beyond one gear tooth (irrespective of any manufacturing tolerances) and not beyond a second tooth. In practice this means that the rotation stop may be set in the region 0t to 2a and With suf?cient clearance to ensure that the effect of manu facturing variability does not take the rotation stop outside of this range. Preferably the rotation stop Will be set toWards the loWer end of the range so that the user does not dial signi? cantly beyond the ?rst gear tooth. A small amount of over rotation is required in order to guarantee that the ratchet arm Will engage. Therefore, the rotational limit of the dose dial Would be set to an angle slightly greater than the angle 0t betWeen the gear teeth. HoWever, this over travel does not contribute to any dose accuracy error of the pen because When the user releases the dose dial the dose spring Will immedi ately rotate the dose dial and drive shaft back until the ratchet arm contacts the gear tooth. Given that the trigger is not pressed at this point then the rotation of the drive shaft does not rotate the gear and hence no medicament is dispensed. A number of options exist for adding rotation restricting fea tures, for example features could be added to the dose dial and the body, or to the drive shaft and the body. [0018] The plunger may be prevented from rotation by thread pitch [3 is 6 m then a 90° rotation Will cause the plunger to advance by a “?xed” increment of 1.5 mm. By features in a reset dial, preferably ?ngers that engage ?ats along the axis of the plunger. Therefore, rotation of the gear appropriate design of the number of gear teeth, and the thread pitch [3 betWeen the gear and the plunger, the nominal volume of the ?xed dose can be altered. A particular advantage of may cause the plunger to advance along its threaded connec devices disclosed herein over prior art devices is that small doses (for example doses<0.05 ml or 5 insulin units) can be delivered With improved dose accuracy. This may be achieved by the combination of large angles 0t and small thread pitch [3. Large angles of 0t mean that any angular error (?x example variability due to the manufacturing tolerances, eg of either gear or ratchet arm) represents only a small percentage of the angle 0t. Combining this With small values of thread pitch [3 Will produce the small advance of plunger necessary for delivering small doses. A further advantage of the larger angle of 0t is that the, preferably torsional, spring, Which Will pro vide the energy required for automatic dose delivery, is loaded over a longer movement. This means that the dialing torque that the user must apply to charge the spring With a given amount of energy is reduced compared to a device With a smaller value of 0t. [0016] As mentioned, if the user fails to rotate the dose dial by an angle suf?cient to engage the ratchet arm of the drive shaft, then the dose Will not be set. The dose spring Will rotate the dose dial and drive shaft back to the Zero unit position of the dose dial. Therefore, it Will be impossible for the user to tion to the gear and this dispenses liquid from the medicament container as it moves a piston or bung in the distal direction. If the trigger is released at any point during the delivery of the dose a trigger spring may force the teeth on the trigger to reengage the gear, preferably to reengage outer teeth on the gear, and cause delivery to stop. Pressing the trigger again Will continue the delivery, until the dose has been delivered. [0019] A further advantage of devices disclosed herein over the prior art is that the trigger and gear may be con?gured so that the trigger only needs a single activation to delivery the full “?xed” dose, Without requiring the user to hold the trigger in the active position. Variable dose devices usually have a trigger that can re-engage With the gear at any point that corresponds to a multiple of the smallest dose that the user can dial and deliver, ie if the inner surface of the gear (Which engages the ratchet arm) has 24 gear teeth then the outer surface of the gear (Which engages the trigger) must also have 24 gear teeth. Therefore, once the dose delivery has been initiated the user may release the trigger button at any time during dose delivery and the delivery Will stop. The possibil ity of stopping the dose delivery may lead to dose errors Where the user mistakenly or deliberately delivers an incom plete dose. Apr. 18, 2013 US 2013/0096513 A1 [0020] A further embodiment of the proposed ?xed dose device seeks to improve the usability of the device so that the entire dose is delivered With one push of the trigger, eg a trigger button, irrespective of Whether the trigger is held. This is accomplished by modifying the trigger mechanism. The setting stage. This may use larger sets of muscles, arms and Wri sts rather than ?nger or thumb poWer, and should therefore be easier for patients. Also, the energy is applied before the needle is injected into the body. Therefore, it does not matter if there is some movement or shaking of the device. teeth of the gear and the trigger may be replaced by a set of [0024] male & female features, e. g. teeth and grooves, on the gear & sible that the user does not press the dose dial fully inWards trigger. The features Would be equally spaced around the circumference of the gear and the number of features Would and therefore may deliver an under dose. With a semi-auto matic device of this disclosure the full dose is achieved more match the number of dose setting teeth of the gear/ drive shaft interface. The proposed features Would make it impossible require pressing a manual dose button can cause the user to for the trigger to re-engage the gear until the gear has com pleted its ?xed rotation. Therefore, the user Would press the trigger once to start the delivery, and then the complete dose Would deliver automatically, irrespective of Whether the user holds the trigger or not. Because the trigger may automati cally slide back into place at the end of the dose, e.g. driven by the trigger spring, the user Will receive visual con?rmation that the dose has been fully delivered. In addition the trigger could be designed to click When it re-engages the gear, pro viding audible con?rmation as Well. [0021] After the medicament container has been emptied the user Will be able to reset the plunger to its initial position and a neW medicament container can then be loaded into the device and the device can be reused. [0022] A titrationpen variant of the ?xed dose device of this disclosure is also possible. In this variant the maximum rota tion of the dose is not limited to alloWing the ratchet arm to rotate beyond only one gear tooth. Instead the maximum rotation is designed to alloW the ratchet arm to rotate beyond multiple gear teeth. This number of teeth that the ratchet arm may rotate past Would correspond to the number of “titration” doses that the user desired to set. The titration device may be con?gured to alloW 5 or less, eg 3 or less, titration doses to be set by a user. For example, if the device Were designed such that a 90° rotation of the plunger delivers a dose of 5 units and the gear Was designed With 4 gear teeth then the ratchet arm Would engage a gear tooth every 90°. Setting the maximum rotation of the dose dial to 270° Would enable three different doses to be set by the user: 5 units, 10 units or 15 units. To reach the 15 unit dose the user Would turn the dose dial through 3 clicks of the drive shaft ratchet, corresponding to 270°. HoWever, unlike the single ?xed dose device this could be achieved by the user in a number of steps. It is therefore not necessary to limit total rotation to 1800 (although each sub In prior knoWn manually driven devices it is pos consistently. LikeWise, dose accuracy in prior devices that not behave consistently Where they Will press and hold the button for varying times. Different button hold times alloW different expansion of the bung or piston, e. g. a rubber bung or a rubber piston, Within the medicament container or cartridge and this has the potential to create variability betWeen doses. The semi-automatic feature of devices disclosed herein elimi nates this variable because the dose spring Will Work inde pendently of the user, particularly during the injection. There fore, the forces applied to the bung or piston of the medicament container during dose delivery may be deter mined by the design of the device, irrespective of forces applied to the trigger button. [0025] In the folloWing text, a set of particularly advanta geous aspects of the injection device is provided by making use of numbers to facilitate making references to the respec tive aspects. [0026] 1. A ?xed dose injection device comprising the fol loWing in combination, [0027] a. a housing; and [0028] b. a dose setting/injecting assembly comprising, [0029] i. a rotatable gear having at least tWo teeth offset from an each other at an angle 0t that corresponds to a predetermined ?xed dose of medicament, Where the gear Wheel is ?xed axially in the housing; [0030] a plunger threadedly engaged With the rotatable gear having a pitch [3 that is matched With angle 0t so that the plunger moves distally during dose delivery a dis tance equal to the predetermined dose; [0031] iii. a torsional spring, and a drive shaft; and [0032] iv. a trigger connected to the rotatable gear such that When the trigger is activated the torsional spring unWinds and causes the gear Wheel to rotate through angle 0t and to move the plunger distally to deliver the predetermined ?xed dose. dose dialed should be less than 180°). In the titration embodi ment delivery of doses in betWeen the ?xed titration doses is [0033] 2. The injection device of aspect 1 Wherein a medi cament housing is connected to the housing and holds a prevented in a similar manner as preventing under doses for the ?xed dose device. If the user dials a dose in betWeen the cartridge of medicament. [0034] 3. The injection device of aspect 1 Wherein the angle ?xed titration doses the dose spring Will rotate the dose dial 0t is from about 20 degrees to about 180 degrees. and the drive shaft back until the ratchet arm engages at the [0035] 4. The injection device of aspect 1 Wherein the ratio of ot/[3 is in the range from about 10 to about 20 degrees/mm. [0036] 5. An ?xed dose titration infection device compris next gear tooth, corresponding to the next loWest titration dose. The set dose Would be clearly displayed to the user by the dose dial, so it Would be clear that the higher dose had not ing the folloWing in combination, been set. [0037] [0023] A semi-automatic feature of devices disclosed herein has certain advantages over knoWn delivery devices that rely on a user’s manual pressing of an injection button. [0038] b. a dose setting/injecting assembly comprising, This creates dif?culty or even pain for elderly use or those With reduced manual dexterity. Also, any shaking of the hand While pressing the dose button Will cause movement of the device and needle, making the injection more painful. A [0039] a. a housing; and i. a rotatable gear having at least a ?rst, a second, and a third tooth offset from an each other at an angle 0t that corresponds to a predetermined ?xed dose of medi cament, Where the gear Wheel is ?xed axially in the housing; [0040] ii. a drive shaft having a ratchet arm that is con semi-automatic device removes this problem. The user sup ?gured engage either the ?rst, the second or the third plies the energy to provide the injection during the dose tooth during dose setting; Apr. 18, 2013 US 2013/0096513 A1 [0041] iii. a plunger threadedly engaged With the rotat able gear having a of pitch [3 that is matched With angle 0t so that the plunger moves distally during dose delivery a distance equal to one or more multiples of the prede termined dose; [0042] iv. a torsional spring; and [0043] v. a trigger connected to the rotatable gear such that When the trigger is activated the torsional spring unWinds and causes the gear Wheel to rotate through angle 0t and to move the plunger distally to deliver the predetermined ?xed dose; [0044] Features describe above orbeloW in connection With different aspects, embodiments etc. may, of course, be com bined With features described in connection With other aspects, embodiments etc. or With a combination of such features even if such a combination is not explicitly described herein. [0045] The advantages mentioned above as Well as other advantages of various aspects of the present disclosure Will become apparent to those of ordinary skill in the art by read ing the folloWing detailed description, With appropriate ref erence to the accompanying draWings. BRIEF DESCRIPTION OF THE FIGURES [0046] Exemplary embodiments are described herein With reference to the draWings, in Which: [0047] FIG. 1 illustrates one possible embodiment of the “?xed” dose injection device of this disclosure; [0048] FIG. 2 illustrates a cross sectional vieW through the “?xed” dose injection device of this disclosure shoWing a ?xed dose stationary gear, the drive shaft and ratchet arm engaging one of the four teeth on the inner surface of the gear; and [0049] FIG. 3 illustrates possible embodiments of the drive shaft, gear and trigger of the dose setting assembly of this disclosure. DETAILED DESCRIPTION [0050] A schematic cross section of one embodiment of the “?xed” dose injection device 1 of this disclosure is illustrated in FIG. 1. The device comprises tWo main assemblies; a dose setting/inj ecting assembly; and a cartridge holder 4. The device comprises a body 3. The dose setting/inj ecting assem bly may be at least partly retained or housed in body 3. The dose setting and cartridge holder assemblies are similar in design and operation to that described in US. Pat. No. 5,104, 380 With signi?cant differences. One signi?cant difference is that the disclosed device is con?gured to alloW the user to set only a ?xed, predetermined dose, as opposed to a large num ber of different doses. This difference in operation may result from a novel design of gear 10. This novel design of the gear is best vieWed in FIG. 2. [0051] A cartridge 23 is retained in the cartridge holder. A piston 17 may be arranged and movably retained in the car tridge 23. The cartridge 23 expediently holds a medicament Which is to be dispensed from the device 1. The piston 17 may seal the medicament Within the cartridge 23. [0052] The term “medicament”, as used herein, preferably means a pharmaceutical formulation containing at least one pharmaceutically active compound, [0053] Wherein in one embodiment the pharmaceutically active compound has a molecular Weight up to 1500 Da and/ or is a peptide, a proteine, a polysaccharide, a vaccine, a DNA, a RNA, an enZyme, an antibody, a hormone or an oligonucleotide, or a mixture of the above-mentioned phar maceutically active compound, [0054] Wherein in a further embodiment the pharmaceuti cally active compound is useful for the treatment and/or pro phylaxis of diabetes mellitus or complications associated With diabetes mellitus such as diabetic retinopathy, throm boembolism disorders such as deep vein or pulmonary throm boembolism, acute coronary syndrome (ACS), angina, myo cardial infarction, cancer, macular degeneration, in?ammation, hay fever, atherosclerosis and/or rheumatoid arthritis, [0055] Wherein in a further embodiment the pharmaceuti cally active compound comprises at least one peptide for the treatment and/ or prophylaxis of diabetes mellitus or compli cations associated With diabetes mellitus such as diabetic retinopathy. [0056] Wherein in a further embodiment the pharmaceuti cally active compound comprises at least one human insulin or a human insulin analogue or derivative, glucagon-like pep tide (GLP-l) or an analogue or derivative thereof, or exedin-3 or exedin-4 or an analogue or derivative of exedin-3 or exe din-4. [0057] Insulin analogues are for example Gly(A21), Arg (B31), Arg(B32) human insulin; Lys(B3) Glu(B29) human insulin; Lys(B28), Pro(B29) human insulin; Asp(B28) human insulin; human insulin, Wherein praline in position B28 is replaced by Asp, Lys, Leu, Val or Ala and Wherein in position B29 Lys may be replaced by Pro; Ala(B26) human insulin; Des(B28-B30) human insulin; Des(B27) human insulin and Des(B30) human insulin. [0058] Insulin derivates are for example B29-N-myristoyl des(B30) human insulin; B29-N-palmitoyl-des(B30) human insulin; B29-N-myristoyl human insulin; B29-N-palmitoyl human insulin; B28-N-myristoyl LysB28ProB29 human insulin; B28-N-palmitoyl-LysB28ProB29 human insulin; B30-N-myristoyl-ThrB29LysB30 human insulin; B30N palmitoyl-ThrB29LysB30 human insulin; B29-N-(N-palmi toyl-Y-glutamyl)-des(B30) human insulin; B29-N-(N-litho cholyl-Y-glutamyl)-des(B30) human insulin; B29-N-(uu carboxyheptadecanoyl)-des(B30) human insulin and B29-N (uu-carboheptadecanoyl) human insulin. [0059] Exendin-4 for example means Exendin-4(1-39), a peptide of the sequence HiHis4GlyiGlu4GlyiThri PheiThriSeriAspiLeuiSeriLys4GlniMeti Glu4Glu4GluiAlaiValiArgiLeuiPheiIlei GluiTrpiLeuiLysiAsn4Gly4GlyiProiSeri Ser4GlyiAlaiProiProiProiSeriNHZ. [0060] Exendin-4 derivatives are for example selected from the folloWing list of compounds: Hi(Lys)4-des Pro36, des Pro37 Exendin-4(1-39)-NH2, Hi(Lys)5-des Pro36, des Pro37 Exendin-4(1-39)-NH2, des Pro3 6 [Asp28] Exendin-4(1-3 9), des Pro36 [IsoAsp28] Exendin-4(1-39), Apr. 18, 2013 US 2013/0096513 A1 des Pro36 [Met(O)14, Asp28] Exendin-4(1-39), des Pro3 6 [Met(O) 14, lsoAsp28] Exendin-4(1-3 9), des Pro3 6 [Trp(O2)25, Asp28] Exendin-4(1-3 9), des Pro36 [Trp(O2)25, lsoAsp28] Exendin-4(1-39), des Pro36 [Met(O)14 Trp(O2)25, Asp28] Exendin-4(1-39), des Pro36 [Met(O)14 Trp(O2)25, lsoAsp28] H-Asn-(Glu)5 -des Pro36, Pro37, Pro3 8 [Met(O) 14, Asp28] Exendin-4(1-3 9) -NH2, des Pro3 6, Pro37, Pro3 8 [Met(O)14, Asp28] Exendin-4(1-3 9)-(Lys)6-NH2, H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exen din-4(1-39)-(Lys)6-NH2, Exendin-4(1-3 9); or des Pro3 6 [Asp28] Exendin-4(1-3 9), H-Asn-(Glu)5 des Pro3 6, Pro37, Pro3 8 [Met(O) 14, Asp28] des Pro36 [lsoAsp28] Exendin-4(1-39), H-Lys6-des Pro36 [Met(O)14, Trp(O2)25, Asp28] Exendin Exendin-4(1-3 9)-(Lys)6-NH2, des Pro36 [Met(O)14, Asp28] Exendin-4(1-39), des Pro3 6 [Met(O) 14, lsoAsp28] Exendin-4(1-3 9), des Pro3 6 [Trp(O2)25, Asp28] Exendin-4(1-3 9), des Pro3 6 [Trp(O2)25, Asp28] Exendin-4(1-3 9), des Pro36 [Met(O)14 Trp(O2)25, Asp28] Exendin-4(1-39), des Pro36 [Met(O)14 Trp(O2)25, lsoAsp28] 4(1-39)-Lys6-NH2, H-des Asp28 Pro3 6, Pro37, Pro3 8 [Met(O)14, Trp(O2)25] Exendin-4(1-3 9) -NH2, H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Asp28] Exen din-4(1-39)-NH2, H-Asn-(Glu)5 -des Pro3 6, Pro37, Pro3 8 [Met(O)14, Trp (O2) Exendin-4(1-3 9), 25, Asp28] Exendin-4(1-3 9)-NH2, [0061] wherein the group -Lys6-NH2 may be bound to the C-terminus of the Exendin-4 derivative; Exendin-4(1-39)-(Lys)6-NH2, or an Exendin-4 derivative of the sequence H-(Lys)6-des Pro36 [Asp28] Exendin-4(1-39)-Lys6-NH2, des Asp28 Pro36, Pro37, Pro38Exendin-4(1-39)-NH2, H-(Lys)6-des Pro36, Pro38 [Asp28] Exendin-4(1-39)-NH2, H-Asn-(Glu)5des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1-3 9) -NH2, des Pro36, Pro37, Pro3 8 [Asp28] Exendin-4(1-3 9)-(Lys)6-NH2, H-(Lys)6-des Pro36, Pro37, Pro38 [Asp28] Exendin-4(1 39)-(Lys)6-NH2, H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Asp28] Exendin-4 (1-39)-(Lys)6-NH2, H-(Lys)6-des Pro36 [Trp(O2)25, Asp28] Exendin-4(1-39) Lys6-NH2, H-des Asp28 Pro36, M037, Pro38 [Trp(O2)25] Exendin-4(1-3 9) -NH2, H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(O2)25, Asp28] Exendin-4(1-3 9) -NH2, H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Trp(O2)25, Asp28] Exendin-4(1-3 9) -NH2, des Pro36, Pro37, Pro38 [Trp(O2)25, Asp28] Exendin-4(1-3 9)-(Lys)6-NH2, H-(Lys)6-des Pro36, Pro37, Pro38 [Trp(O2)25, Asp28] des Pro36, Pro37, Pro38 [Met(O)14, Trp(O2)25, Asp28] H-(Lys)6-des Pro36, Pro37, Pro38 [Met(O)14, Trp(O2)25, Asp28] Exendin-4(S1-39)-(Lys)6-NH2, H-Asn-(Glu)5 -des Pro3 6, Pro37, Pro3 8 [Met(O)14, Trp (O2) 25, Asp28] Exendin-4(1-3 9)-(Lys)6-NH2; [0063] or a pharmaceutically acceptable salt or solvate of any one of the afore-mentioned Exedin-4 derivative. [0064] Hormones are for example hypophysis hormones or hypothalamus hormones or regulatory active peptides and their antagonists as listed in Rote Liste, ed. 2008, Chapter 50, such as Gonadotropine (Follitropin, Lutropin, Choriongona dotropin, Menotropin), Somatropine (Somatropin), Desmo pressin, Terlipressin, Gonadorelin, Triptorelin, Leuprorelin, Buserelin, Nafarelin, Goserelin. [0065] A polysaccharide is for example a glucosaminogly cane, a hyaluronic acid, a heparin, a loW molecular Weight heparin or an ultra loW molecular Weight heparin or a deriva tive thereof, or a sulphated, eg a poly-sulphated form of the above-mentioned polysaccharides, and/or a pharmaceuti cally acceptable salt thereof. An example of a pharmaceuti cally acceptable salt of a poly-sulphated loW molecular Weight heparin is enoxaparin sodium. [0066] Pharmaceutically acceptable salts are for example acid addition salts and basic salts. Acid addition salts are e.g. HCl or HBr salts. Basic salts are e.g. salts having a cation selected from alkali or alkaline, e.g. Na+, or K+, or Ca2+, or an ammonium ion N+(R1)(R2)(R3)(R4), Wherein R1 to R4 Exendin-4(1-3 9)-(Lys)6-NH2, independently of each other mean hydrogen, an optionally substituted C1-C6-alkyl group, an optionally substituted H-Asn-(Glu)5-des Pro36, Pro37, Pro38 [Trp(O2)25, Asp28] C2-C6-alkenyl group, an optionally substituted C6-C10-aryl Exendin-4(1-3 9)-(Lys)6-NH2, group, or an optionally substituted C6-C10-heteroaryl group. H-(Lys)6-des Pro36 [Met(O)14, Asp28] Exendin-4(1-39) Lys6-NH2, Pro37, Pro38 des Met(O)14 Asp28 Pro36, Exendin-4(1-3 9) -NH2, [0062] H-(Lys)6-desPro36, Pro37, Pro38 [Met(O)14, Asp28] Exendin-4(1-39)-NH2, Further examples of pharmaceutically acceptable salts are described in “Remington’s Pharmaceutical Sciences” 17. ed. Alfonso R. Gennaro (Ed.), Mark Publishing Company, Eas ton, Pa., U.S.A., 1985 and in Encyclopedia of Pharmaceutical Technology. [0067] Pharmaceutically acceptable solvates are for example hydrates. Apr. 18, 2013 US 2013/0096513 A1 [0068] A disposable injection needle assembly (not shown) on piston 17 inside cartridge 23. This causes the pressure in is attached to hub 2, Which preferably has external threads that the cartridge to increase and liquid medicament is dispensed engage similar pitch threads inside a female hub on the dis from the distal end of the cartridge into an attached needle. posable needle assembly. The setting of the ?xed dose Within injection device 1 is controlled by the interaction of the drive shaft 9, Which is preferably permanently connected to dose dial 5, and gear 10, Which is expediently slidably engaged With trigger 6. During dose setting, gear 10 is prevented from The pitch [3 may be 6 mm, for example, resulting in an axial displacement of the plunger by 1.5 mm When the gear is rotated by the angle 0t of 90°. If the trigger 6 is released at any point during the delivery of the set dose, teeth 15 and 16 Will re-engage stopping the rotation of gear 10 and thus stopping the delivery of medicament. When the trigger 6 is being held by the user in the activated position a trigger spring 8 may tend to push the trigger 6 into re-engagement With gear teeth rotation by the interaction of components on the gear and trigger. Drive shaft 9 features a ratchet arm 12 (see FIG. 2) that engages teeth 11 on an inner surface 14 of gear 10. Each tooth 11 is separate by an angle 0t from the next tooth, shoWn 15 on the outside of the gear 10. When the trigger 6 is released in FIG. 2 as 90°. Each tooth of the gear corresponds to one predetermined ?xed dose. By appropriate design of the num by the user, the trigger spring 8 Will re-engage trigger and gear. Pres sing the trigger again Will continue the delivery until ber of gear teeth, as described above, and the thread pitch betWeen the gear and the plunger, the nominal volume of the medicament is complete When the dose dial 5 reaches a rota ?xed dose can be altered. tional stop (not explicitly shoWn) With respect to the body 3. [0069] This stop position corresponds to the “Zero dose selected” position of the dose dial 5. Movement of the plunger 7 is achieved by energy stored in a helical torsional spring 18, Which is tWisted as the dose of medicament to be injected from the cartridge 23 is set by rotation of dose dial 5. The dose dial can turn about a graduated sleeve or the drive shaft and may contain printed graduations that correspond to the ?xed dose. Body 3 can have a lens or one or more WindoWs (not explicitly shoWn) through Which graduations on the sleeve or shaft can be read to shoW that the ?xed dose has been set. [0070] When dialing a dose the user rotates dose dial 5, Which causes the connected drive shaft 9 and ratchet arm 12 to rotate and click over one of the teeth 11 in gear 10. The user may rotate the dose dial by the angle 0t or an angle Which is slightly greater than 0t. The user may be prevented from rotating the dose dial 5 by 2a by a rotational stop (not explic itly shoWn) Which may be provided in the body, for example. Thus, setting of a dose exceeding the ?xed dose may be prevented. Alternatively, instead of providing a ?xed dose device Where only one dose may be dialed, a titration device may be provided as described further above, Which is designed such that a limited number, eg 5 or less, preferably 3 or less, titration doses may be dialed by the user. Rotating the dose dial also stores torsional energy Within the corre sponding dose spring 18 that is fastened to the dose dial. This stored torsional energy is used to automatically deliver the medicament dose When trigger 6 is pressed or otherWise activated causing the spring 18 to unWind and returning the dose dial 5 and the drive shaft 9 to the initial Zero position. When the trigger 6 is activated, teeth 16 disengage from teeth 15 on gear 10 and the gear becomes free to rotate With drive shaft 9 (see FIG. 3). [0071] The stored energy of the dose spring 18 drives the drive shaft 5 in rotation, Which in turn rotates the gear 10. The gear 10 has a central threaded opening 22 that is in threaded all of the dialed units have been delivered. The injection of [0072] If the user fails to rotate the dose dial by an angle suf?cient to engage the ratchet arm 12 of the drive shaft 9, then the dose Will not be set. The dose spring Will rotate the dose dial and drive shaft back to the Zero unit position of the dose dial. Therefore, it Will be impossible for the user to set any dose beloW the “?xed dose” determined by the spacing of the gear teeth [0073] Upon injection, axial movement of the plunger 7 along the quick pitch thread, Which may be provided in open ing 22, accompanies rotation of gear 10 and drive shaft 9 since the plunger 7 cannot rotate in the reset dial 13. Thus, the plunger is driven into the cartridge. expelling the ?xed dose of medicament from the cartlidge. The injection of medicament is complete When dose dial 5 reaches a rotational stop (not explicitly shoWn) With respect to the body 3. The stop position corresponds to the “Zero dose selected” position of dose dial 5. When the dose dial 5 reaches its rotational stop on the pen body, the plunger remains part-Way along the inside of the cartridge. The procedure can be repeated until the cartridge is exhausted, after Which the cartridge can be replaced by removing the cartridge holder from the dose dialing assem bly. [0074] In some cases, the requirement to hold doWn the trigger until the injection is complete may be considered undesirable and therefore an alternate embodiment Would include a con?guration that prevents the trigger from reen gaging With the gears even though the user releases the trig ger. This may, for example, be accomplished by including a detent mechanism Whereby pressing the trigger causes it to bump-over a detent so that it Will remain in the forWards position even if the user releases the trigger, eg a trigger button. The detent could be designed to disengage only When engagement With plunger 7. The plunger is prevented from the drive shall or dose dial have returned to their initial, Zero, rotation by features in reset dial 13. Preferably, the reset dial positions. This Would enable the complete injection to be delivered With only a single press of the trigger (Without the need to hold the trigger forWards). A further advantage Would 13 contains tWo protrusions (not shoWn) that consist of opposed ?ats that engage tWo longitudinal grooves on opposed sides along the length of the drive plunger 7. When the corresponding cartridge holder 4 is attached to the body 3, the reset dial 13 is prevented from rotation relative to the body 3. Thus, When the cartridge holder 4 is connected to the body 3, rotation of the drive plunger 7 is prevented. Because the be that the return of the trigger to its initial position Would give the user additional visual and audible feedback to indi cate that the dose is complete. [0075] Alternatively or additionally, the ?xed dose device may be con?gured so that the entire dose is delivered With one drive plunger 7 cannot rotate and the gear 10 cannot move push of the trigger 6, eg a trigger button, irrespective of axially, the rotation of the gear 10 causes the drive plunger 7 Whether the trigger is held or detented as described above. to advance along its thread having pitch [3 that is engaged With This may be accomplished by modifying the trigger mecha the thread in gear 10 in an axial distal direction exerting force nism. The teeth 15 of the gear 10 and the teeth 16 of the trigger Apr. 18, 2013 US 2013/0096513 A1 6 may be replaced by a set of male and female features (not explicitly shown), e.g. teeth and grooves, on the gear 10 and on the trigger 6. The features may be equally spaced around the circumference of the gear 10. The number of features preferably matches the number of gear teeth 11. These fea tures are preferably designed such that it is impossible for the trigger 6 to re-engage the gear 10 until the gear has completed its ?xed rotation for delivering the ?xed dose. Therefore, the user Would press the trigger 6 once to start the delivery, and then the complete dose may be delivered automatically, irre spective of Whether the user holds the trigger 6 or not. Because the trigger 6 may automatically slide back into place at the end of the dose, e.g. driven by the trigger spring 8, the user Will receive visual con?rmation that the dose has been fully delivered. [0076] The reset dial 13 not only prevents the plunger from iv. a trigger connected to the rotatable gear such that When the trigger is activated the torsional spring unWinds and causes the rotatable gear to rotate through angle 0t and to move the plunger distally to deliver the predetermined ?xed dose; and v. a rotation stop that limits the rotation of the drive shaft, preventing a rotation beyond more than one tooth of the rotatable gear. 9: The injection device of claim 8 Wherein a medicament housing is connected to the housing and holds a cartridge of medicament. 10: The injection device of claim 8 Wherein the angle 0t is from 20 degrees to 180 degrees. 11: The injection device of claim 8 Wherein the ratio of 01/[3 is in the range from 10 to 20 degrees/mm. 12: The injection device of any of claim 8 Wherein the pitch rotation during that movement, but it also acts as a means ?x [3 ofa thread on the plunger is from 1.5 mm to 12 mm. retracting the drive plunger 7 back into the body 3 thus reset ting the device. When the cartridge holder 4 is attached to the body 3 the reset dial 13 is restrained against rotation in the comprises a ratchet arm that engages one of the teeth during 13: The injection device of claim 8 Wherein the drive shaft dose setting. body 3 by a locking member (not explicitly shoWn) ?tted into 14: The injection device of claim 8 Wherein the drive shaft the reset dial 13. When the cartridge holder 4 is removed the locking member is unrestrained and alloWs rotation of the reset dial 13. The drive plunger 7 may be prevented from rotation relative to the reset dial 13 by the engagement of reset comprises a ratchet arm that is con?gured to engage one of the teeth during dose setting. 15: A ?xed dose titration injection device comprising the folloWing in combination, dial protrusions and drive plunger longitudinal moves (not a. a housing; and explicitly shoWn). The drive plunger 7 is threadedly engaged b. a dose setting and injecting assembly comprising, to the gear 10, Which is ?xed in rotation relative to the body 3 by the engagement of gear teeth 15 and trigger teeth 16. i. a rotatable gear having at least a ?rst, a second, and a third tooth offset from each other at an angle 0t that Rotation of the reset dial 13 causes the drive plunger 7 to rotate Which causes it to Wind up the thread in the gear 1 0, thus corresponds to a predetermined ?xed dose of medi cament, Where the rotatable gear is ?xed axially in the retracting the drive plunger 7 back into the body 3. [0077] Exemplary embodiments of the present disclosure have been described. Those skilled in the art Will understand, hoWever, that changes and modi?cations may be made to these embodiments Without departing from the true scope and spirit of the subject matter, Which is de?ned by the claims. 1-7. (canceled) 8: A ?xed dose injection device comprising the folloWing in combination, a. a housing; and b. a dose setting and injecting assembly comprising, i. a rotatable gear having at least tWo teeth offset from each other at an angle 0t that corresponds to a prede termined ?xed dose of medicament Where the rotat able gear is ?xed axially in the housing; ii. a plunger threadedly engaged With the rotatable gear having a pitch [3 that is matched With angle 0t so that the plunger moves distally during dose delivery a distance corresponding to the predetermined ?xed dose; iii. a torsional spring, and a drive shaft Wherein the torsional spring rotates the drive shaft back to a Zero position if the drive shaft is rotated by an angle smaller than 0t; housing; ii. a drive shaft having a ratchet arm that is con?gured to engage either the ?rst, the second or the third tooth during dose setting; iii. a plunger threadedly engaged With the rotatable gear having a pitch [3 that is matched With angle 0t so that the plunger moves distally during dose delivery a distance corresponding to one or more multiples of the predetermined ?xed dose; iv. a torsional spring Wherein the torsional spring rotates the drive shaft back until the ratchet arm engages at the next gear tooth, corresponding to the next loWer dose, if a dose is dialled in betWeen tWo ?xed titration doses; and v. a trigger connected to the rotatable gear such that When the trigger is activated the torsional spring unWinds and causes the rotatable gear to rotate through one or more multiples of angle 0t and to move the plunger distally to deliver the one or more mul tiples of the predetermined ?xed dose, vi. a rotation stop that limits the rotation of the drive shaft, preventing a rotation beyond a limited number of teeth of the rotatable gear. * * * * *