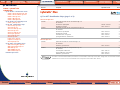
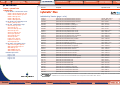
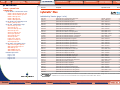
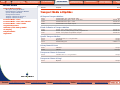
Download Pre-Poured Media – Plates - bioMérieux Nordic Countries website
Transcript
HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List NORDIC CLINICAL PRODUCT LIST 2013 BACTERIOLOGY IMMUNOASSAYS MOLECULAR DIAGNOSTICS Welcome to the bioMérieux Clinical PDF Product List. Use the Head Tabs at the top of each page to skip to each section, then click the titles in the Tab Menu to find each product group. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 1 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Our Principles Customers come First We must satisfy our customers - clinicians and laboratory professionals with high quality, innovative products and services to protect and improve the health of patients and consumers worldwide. Employees are our Greatest Asset We must respect and recognise the work and the ideas of all employees, no matter what their job. We work together towards a common goal: satisfy our customers. We must provide employees with the training and resources they need to achieve this. We Belong to a Community We have social, environmental and economic responsibilities towards the local communities where our sites are located. We also have responsibilities towards the global community as a partner in public health. Our Shareholders are Partners We must communicate company information in a transparent, thorough and timely manner. We must grow bioMérieux’s sales and ensure that shareholder investments provide a proper return. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 2 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Sales Orders & Enquiries Our telephone lines are open Monday to Friday (except Bank Holidays) according to the info of office addresses below: Denmark Finland Norway Sweden Order Team Order Team Order Team Order Team Telephone: 70 10 84 02 Telephone: 09 8545 6040 Telephone: 2337 5552 Telephone: 031 68 84 96 Fax: 70 10 84 01 Fax: 09 8545 6045 Fax: 2337 5551 Fax: 031 68 48 48 [email protected] [email protected] [email protected] [email protected] General Enquiries General Enquiries General Enquiries General Enquiries Telephone: 70 10 84 00 Telephone: 09 8545 600 Telephone: 2337 5550 Telephone: 031 68 84 90 Fax: 70 10 84 01 Fax: 09 8545 6045 Fax: 2337 5551 Fax: 031 68 48 48 [email protected] [email protected] [email protected] [email protected] Opening Hours Opening Hours Opening Hours Opening Hours Monday to Thursday Monday to Friday Monday to Friday Monday to Friday from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm bioMérieux Denmark ApS bioMérieux Suomi Oy bioMérieux Norge AS bioMérieux Sweden AB Smedeholm 13C Konalantie 47C Ulvenveien 75A, 3 etg. Hantverksvägen 15 2730 Herlev 00390 Helsinki 0581 Oslo 43633 Askim Fridays 8.30am to 3.00pm Quotations If you have received a quotation from your representative please have the quote reference to hand when placing an order to ensure the correct pricing is set up. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 3 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Customer Support Support enquiry e-mails are logged by our Customer Support team. Our telephone hotlines are open Monday to Friday (except Bank Holidays) between the times listed below. Application Support Technical Support To help with all of your enquiries we have a team of in-house or Our field Service Engineers install equipment, perform preventative field Application Specialists maintenance (servicing) and provide emergency breakdown cover If our team can deal with your enquiry over the telephone they will in your laboratory endeavour to do so The service team perform instrument pre-delivery inspections, If this is not possible or you require more hands-on help with an repairs and airIDEAL® air flow calibrations evaluation, validation or training then your enquiry will be escalated to our field Application Specialists who may then arrange to visit your laboratory Denmark Finland Norway Sweden Hotline: 44 508 408 Hotline: 09 8545 6020 Hotline: 2337 5555 Hotline: 031 68 58 58 Opening Hours Opening Hours Opening Hours Opening Hours Monday to Thursday Monday to Friday Monday to Friday Monday to Friday from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm bioMérieux Denmark ApS bioMérieux Suomi Oy bioMérieux Norge AS bioMérieux Sweden AB Smedeholm 13C Konalantie 47C Ulvenveien 75A, 3 etg. Hantverksvägen 15 2730 Herlev 00390 Helsinki 0581 Oslo 43633 Askim Fridays 8.30am to 3.00pm E-mail address for all Nordic Countries [email protected] Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 4 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List mybiomerieux.com Quality Control Certificates bioMérieux customer portal www.mybiomerieux.com is designed Our pre-poured media products must be passed by our Quality to be part of the future e-platform. Control Departments and a certificate produced before they are www.mybiomerieux.com offers an easy account creation and hosts Technical Library as well as coming applications like validation packages, distance learning, and others. Once you have registered, you can access: released for sale. The QC Certificates for these and other products can be accessed directly via www.mybiomerieux.com. You can search for these using the product name, reference number or the lot number. Expiry Dates Package Inserts For pre-poured media and kits, the expiry date for a specific batch is on Quality Control Certificates the top of the QC Certificate. Product can be used on the day of expiry. Material Safety Data Sheets in English (more about MSDS in Nordic languages in "Quality and Environment") Thermal Shock Validations Instrument Manuals Validation guides and addenda LyfoCults® Plus To obtain Certificates of Quality for our LyfoCults Plus products, visit www.pmlmicro.com To find Instrument user manuals or validation addenda, choose BROWSE BY SYSTEMS from the BROWSE BY PRODUCT RANGE drop down menu and this will bring you to a list of our instruments. Technical Library page for www.mybiomerieux.com QC Certificate Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 5 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List History bioMérieux Over a Century in the Service of Global Public Health PAGE 1 OF 2 PAGE 2 OF 2 The commitment of the Mérieux family to work in the field of biology goes back to 1897, with the establishment by Marcel Mérieux, a pupil of Louis Pasteur and Emile Roux, of Institut Mérieux. This marks the start of an extraordinary adventure in both biology and industry, which was to have a profound impact on vaccinology and on in vitro diagnostics at the global level. Initially under the direction of Doctor Charles Mérieux and later of Alain Mérieux, Institut Mérieux was to become a world leader in the field of human and veterinary vaccines. It gave birth to many bodies which are still major players today in the public health arena: In human medicine, Pasteur Mérieux Connaught which later became Aventis Pasteur and then Sanofi Pasteur In veterinary medicine, IFFA (Institut Français de Fièvre Aphteuse) which became Rhône Mérieux, and later Merial In parallel to this work on vaccines, in 1963 Alain Mérieux set up bioMérieux to work on in vitro diagnostics. The family's corporate holding was later enlarged through the acquisition of Transgene, working in immunotherapy, and then the American industrial control company Silliker, followed by ABL (a North American company researching into virology). Marcel Mérieux, a student of Louis Pasteur, creates the Institut Mérieux (Year 1897). Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 6 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING CULTURE MEDIA IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List History bioMérieux Over a Century in the Service of Global Public Health PAGE 2 OF 2 PAGE 1 OF 2 The name of Mérieux Alliance was chosen to reflect the full scope of Institut Mérieux has thus set up Mérieux Développement, a company these activities. Under the impetus of Alain Mérieux and his sons, investing in the area of health. 2010 has seen the Institute increase its Christophe, Rodolphe and Alexandre, the group developed further, commitment to Nutrition/Health with the structuring of Mérieux fostering a policy of scientific innovation and international openness by NutriSciences grouping together Silliker, Biofortis and Bioagri. It has developing a network of sites all over the world. also created IMAccess, a company dedicated to biology without borders between the North and South. In 2009, Mérieux Alliance is changing its name and again taking up the standard of “Institut Mérieux” to communicate its vision of biology and to take on the new challenges in global public health. Human Health 2000 > Sanofi Pasteur* Vaccines - Biotherapies 2000 > Aventis Pasteur* 1994 > Pasteur Mérieux Connaught* Nutrition/Health Pasteur Mérieux Sérums & Vaccins 1985 > Pasteur Vaccins Diagnosis 1897 Institut Biologique Mérieux 2009 DIAGNOSTIC 1963 BD Mérieux 1974 bioMérieux 1998 Silliker 1994 Transgene 2010 Mérieux NutriSciences 2001 ABL Merieux Alliance 1967 Fondation Mérieux 2001 Fondation Christophe et Rodolphe Mérieux 2010 IMAccess Mérieux Développement Immunotherapy 1947 > Creation of IFFA Animal Health Vaccines - Medicaments 1968 > IFFA Mérieux 1983 > 1994 > Rhône-Mérieux 1997 > 2004 > Mérial* * Companies founded by the Mérieux family but no longer part of Institut Mériuex since 1994. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 7 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING CULTURE MEDIA IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Quality & Environment QMS QA QC MSDS Quality Policy Quality by Design At bioMérieux, we never forget that every day, somewhere in the world, We have our minds set on doing better every day and when it comes a clinician makes a decision for a treatment, or a food product is released to our Quality Policy, we adopt a holistic approach. Our goal is quality for consumption, based on the quality of a test we are providing. by design in everything we do: our aim is not to create quality by Our tests must provide an optimum level of performance because our customers are themselves responsible for the quality of the test results checking, but quality right from the start by integrating it into every aspect of our work. they provide to those who depend on them. This is why we consider We have a Global Quality Management System to control our quality to be everybody’s responsibility. processes and manage risk worldwide, certified against ISO 9001. This bioMérieux’s Quality Policy guides our internal processes and drives good business outcomes and efficiencies. In line with regulatory requirements, it also draws upon input from the company’s sites and subsidiaries worldwide to ensure that the policy is understood and embraced by all employees. approach helps assure fulfilment of regulatory requirements. It leads to decreased production cycle times, reduced product and service nonconformities and an optimised supply chain. Products can thus be launched rapidly on the market and processes can be adapted to satisfy the needs of our customers. Quality is Everybody’s Responsibility Quality is not the job of the Quality Organisation only: it is a shared responsibility by all employees, affecting everybody from R&D and manufacturing to sales and marketing, as well as support functions. Performance indicators engage all employees in the achievement of quality objectives. Continuous improvement initiatives are in place, and best practices are exchanged among the company’s teams worldwide. It is our responsibility to help customers do their job by understanding their needs and making sure that we meet them. Our Quality Policy is key to achieving this. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 8 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING CULTURE MEDIA IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Quality & Environment QMS QA QC MSDS To meet the high expectations of our customers, our Quality Assurance system is designed to ensure the reproducibility of product performance throughout a product's lifecycle. Each new product is validated following stringent performance criteria required by our customers and by regulatory bodies such as the FDA, AFSSAPS and other organisations such as LNE/G-MED, DNV and specified by pharmacopoeias, food accreditation bodies and ISO standards. Quality Regulations Inspections at Our Production Sites & Subsidiaries Our Quality Assurance and Regulatory Affairs Departments must 25–30 customer audits a year approve all products before release. All development and validation International health authorities such as the FDA and AFSSAPS documentation is archived and can be accessed during an audit. ISO 9000 family and ISO 13485 audits The following are requirements applicable to all bioMérieux entities: ISO 9001:2008, Quality management systems – Requirements Raw Material Management ISO 13485:2003, Medical-Devices – Quality Management Strict selection and control of raw materials to maintain the Systems – Requirements for regulatory purposes reproducibility of performance between batches ISO 14971:2007, Application of Risk Management to Medical Devices Control of the supply chain for raw materials FDA cGMP 21 CFR Part 820 (current Good manufacturing Raw materials are blended for each product Practises) Quality System Regulation Full traceability from raw material to finished product Culture media ranges manufactured for use in the pharmaceutical TSE/BSE Status Certification industry are compliant with the US/EU/J pharmacopoeia requirements All our products meet, as applicable, the requirements of: European Medical Device Directive 93/42/EEC European In Vitro Diagnostic Directive 98/79/EC US/EP/JP Pharmacopoeia Our subsidiaries are compliant with: European Packaging and Packaging Waste Directive 94/62/EC Waste Electrical and Electronic Equipment Directive 2002/96/EC Culture Media Production Plate production in clean rooms with environmental monitoring and controls Filling areas are operated to ISO 5 classification Special manufacture for customer specified formulations, filling volumes and presentations for plates and bottles Copies of our certificates can be obtained upon request. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 9 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING CULTURE MEDIA IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Quality & Environment QMS QA QC MSDS Performance & Shelf-life Evaluated for each product according to: Quality Control Microbiological and physical criteria for raw materials and EN 13640:2002 Stability Testing of in-vitro diagnostic reagents final product EN 1322:1999 In-vitro Diagnostic Devices – Culture Media for Includes: growth promotion (recovery rates, colony morphology), Microbiology – Performance Criteria for Culture Media pH, colour, agar strength Using validated methods Pharmaceutical culture media are tested using microbial strains Inter-lot and Intra-lot variability analysed to prevent any drift in defined by the USP/EP/JP performance (USP 1117) >50% recovery rate compared with a gold standard media Stability testing: Unique ≥ 75% recover rate compared with a previously passed In real-time on 3 batches batch of the same media for all 3P™ media Includes thermal shocks to guarantee shipment and Non-sterile pharmaceutical testing culture media complies with temperature variations will not impact on performance the harmonised chapters of the pharmacopoeias: Pharmaceutical culture media – wild and pharmacopoeia Microbiological examination of non-sterile products: microbial strains tested in development enumeration tests (EP 2.6.12, USP 61, JP 35.1) Routinely re-evaluated after launch Microbiological examination of non sterile products: tests for Extends to beyond the expiry given so that media can specified microorganisms (EP 2.6.13, USP 62, JP 35.2) be used on the last day of shelf-life with no impact on performance Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 10 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING CULTURE MEDIA IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Quality & Environment QMS QA QC MSDS Material Safety Data Sheets An MSDS provides lab personnel with procedures for handling or working with dangerous products in a safe manner, and include information such as physical data, toxicity, health effects, storage, disposal, protective equipment, and waste-handling procedures. bioMérieux reagents conform to all European and national regulations applicable to chemical substances that might have an impact on health or environment. When required MSDS exist for our products. They can easily be retrieved from www.mybiomerieux.com under Technical Library in English. MSDS in Danish, Finnish, Norwegian and Swedish can be found at www.ecoonline.com where customer own report creation tools also exist. To find our Nordic MSDS easily they can be searched by article number and language wanted. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 11 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List bioMérieux Goes Green – Environmental Commitment Five Key Areas Throughout the world, bioMérieux is going green. On each site, in every subsidiary, a green champion is in charge of ensuring we achieve our environmental goals in the following five areas of focus. Energy Waste Reduce our Consumption of Non-Renewable Energy Sources. Minimise Adverse Impacts on Health and the Environment. Conscious that continued use of non-renewable energy sources will We have been optimising our waste management for a number of cause greater environmental problems, we want to use energy as years, reducing the weight of landfill waste, recycling and sorting responsibly and efficiently as possible. We have implemented an non-hazardous waste. Specialised companies handle hazardous waste. eco-efficient program focused on three major areas: reduce consumption, increase equipment efficiency, experiment with alternative energy sources. For examples of how we are achieving our goal, click here. Water Conserve Water Through Good Management of Resources. Water is the resource we use the most in our manufacturing processes. To reduce consumption, we favour closed loop circuits and are actively replacing once-through cooling systems. For examples of how we are achieving our goal, click here. Emissions Minimise Adverse Impacts on Health and the Environment. Global warming due to greenhouse gas emissions is a major problem. Because of the close link between CO2 emissions and global warming, we are focusing our efforts on high CO2 emission sources that have a significant impact on the atmosphere. For examples of how we are achieving our goal, click here. For examples of how we are achieving our goal, click here. Paper Reduce Paper Usage in Processes, Packages and Products. As we are constantly growing, we are faced with the issue of increased paper consumption. We are meeting this challenge by changing our product packaging and recycling paper. For examples of how we are achieving our goal, click here. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 12 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Terms & Conditions PAGE 1 OF 5 1. PAGE 2 OF 5 PAGE 3 OF 5 PAGE 4 OF 5 4. ORDERS 1.1 1.2 PAGE 5 OF 5 PRICES To avoid errors, each order should specify: 4.1 Prices of Products are quoted net ex-works (Incoterms 2010 ICC). - product name and reference number 4.2 VAT shall be applicable to all sales for re-export unless evidence of exemption is - the addresses for delivery and invoicing supplied at the date of the order. Reagents, disposables and accessories prices bioMérieux's acceptance of any order shall be conditional upon creditworthiness are invoiced at the price in force at the date of delivery and in accordance to of the Buyer. In particular, bioMérieux reserves the right to suspend the order if agreed prices with bioMérieux Sales department. the situation of the Buyer visibly presents a risk with regards to the recovery of 4.3 the outstanding debts of bioMérieux, or in case of unavailability of the Product. Unless otherwise expressly agreed by the Parties, all shipment costs and expenses are prepaid by bioMérieux and re-invoiced to the Buyer, even for partial shipments. Concerning the participation in handling costs and delivery prices: - bioMérieux charges an administration fee on each order - bioMérieux charges a dangerous goods fee for shipments that include 5. INVOICING 5.1 products with UN numbers - Products are invoiced on the shipping date, at the price in force at the date of order. Unless otherwise informed by the Buyer, bioMérieux reserves its right to Express delivery costs, when such deliveries are asked by the customer, establish a deferred invoicing of the Products. are charged to the customer 5.2 Delay by the Buyer in challenging the invoiced amount within a period of 30 days from the date of the invoice shall be deemed acceptance thereof by the Buyer. 2. SUBSCRIPTION CONDITIONS 2.1 Adjustable subscriptions are available for usual and regular consumer goods. 2.2 Notwithstanding the above, subscription shall be required for special products, 6. PAYMENT 6.1 Payment shall be made in cash in advance or, in case of bioMérieux’s express prior 6.2 A penalty is due by Buyer in case of late payment and is to be calculated from consent, 30 days net following the invoicing date, by cheque or by bank transfer. cells, blood and red cells of animals. 2.3 Given the special treatment of these Products, an additional cost per shipment shall be applied. the day following the date of payment stated on the invoice. The amount of such penalty is calculated at the interest rate applied by the European Central 3. DELIVERIES 3.1 Sales of Products shall be made DDP (Delivered Duty Paid, Incoterms 2010 ICC) Bank at its most recent refinancing operation with a ten point increase. 6.3 Failure by the Buyer to fulfil its payment obligations, bioMérieux reserves the right: - to remove immediately all the payment facilities and special commercial special agreement has been made with bioMérieux Sales department. - to suspend or cancel, without notice and compensation, any current order bioMérieux undertakes to provide the Buyer with the Products (instruments, - to require, in order to perform any subsequent delivery, cash payment to the delivery address specified by the customer in the order. The Products will conditions previously granted not be delivered to specific departments or rooms inside the building, unless a 3.2 before each shipment, or any other means of payment chosen reagents and disposables) indicated on the order accepted by bioMérieux. by bioMérieux Products will be delivered and installed as specified in Buyer’s purchase order - accepted by bioMérieux. 3.3 bioMérieux undertakes to deliver goods from stock without delay. In case of temporary unavailability, the customer will receive this information from 6.4 to require immediate payment of the entire outstanding balance Payment should be made by bank transfer to the bank account indicated on the invoice. bioMérieux. The shipment of the outstanding balance of the order shall be done within the frame. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 13 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Terms & Conditions PAGE 1 OF 5 7. PAGE 2 OF 5 PAGE 3 OF 5 PAGE 4 OF 5 LEASE PURCHASE In case of lease purchase, the funding agency shall assume the rights and obligations hereunder as Buyer, and shall guarantee the rights and obligations of the end-user. PAGE 5 OF 5 11. WARRANTIES 11.1 Reagents: bioMérieux’s reagents are intended for in vitro use. The conformance of the reagents to the specifications indicated in the package insert is guaranteed until 8. OWNERSHIP RIGHTS AND PASSING OF RISK 8.1 8.2 All products remain bioMérieux's property until full payment thereof by Buyer. In their expiry date. 11.2 Instrument: the event of non-payment by Buyer, bioMérieux reserves the right to recover or to bioMérieux warrants the Instrument to the Buyer against defects in material and make recover possession of the unpaid goods. bioMérieux shall retain the part of workmanship and defects arising from failure to conform to the user’s manual the price already paid as a compensation for the use of the goods. The promise applicable on the date of installation, and as provided to the Buyer, for the to pay shall not be considered, for the purposes of this provision, as payment. period of one (1) year from the date of installation, when the certificate of Notwithstanding the above, the Buyer is liable for all damage occurring to the installation or equivalent is signed. Products or damage caused by the Products. In accordance with the Incoterms bioMérieux agrees to correct or have corrected by a third party designated by as per Article 3, risk of loss or damage are transferred from bioMérieux to the bioMérieux, either by repair or at its election, by replacement, any such defect Buyer after delivery of such Products to the Buyer. The Buyer shall therefore found on examination to have occurred, when the Instrument has been used ensure that its insurance covers the Products delivered from this time. under normal operating conditions and maintained in accordance with the instructions provided by bioMérieux such as, but not limited to, written 9. TRACEABILITY instructions, package insert and user’s manuals, during such one (1) year The technical specificities of products sold by bioMérieux require compliance with warranty period, provided bioMérieux is promptly notified in writing upon traceability rules. Consequently, bioMérieux reserves the right to sell its Products solely discovery of such defect by the Buyer. to professionals authorized to provide biological analysis results. The Buyer undertakes Disposables and replacement items with a normal life expectancy of less than to comply with all traceability rules which apply to the Products ordered. bioMérieux one (1) year such as, but not limited to, batteries, lamps and tubing are will not be liable for non-compliance with traceability rules after delivery of its products excluded from this warranty. to the site specified by Buyer. bioMérieux warranty is valid to the Buyer’s original site of installation only. Any transhipment VOIDS this warranty unless bioMérieux facilitates the relocation of 10. CLAIMS 10.1 The Buyer shall have checked the incoming parcel and within three (3) days the Instrument. 11.3 Software: from the delivery report to the bioMérieux order department any missing or bioMérieux warrants that the Software (other than the one embedded in the damaged products. Instrument) will be in accordance with the descriptions and specifications in the 10.2 The Buyer shall check the incoming parcel directly on arrival and if any damage is visible from outside it must be written down on the delivery note at arrival. 10.3 Claims must be sent to bioMérieux Customer service as soon as possible documentation accompanying such Software for a period of ninety (90) days from the date of installation under normal use and conditions. During this warranty period, bioMérieux agrees to provide technical support of the Software by correcting upon notice. No product returns shall be accepted without bioMérieux's prior any deficiency of the Software and, in no case, by redesigning the Software. written consent. bioMérieux shall decide, at its sole judgement, if the Products Software developed by and the property of third parties is warranted to be in shall be replaced. accordance with the descriptions and specifications in the documentation accompanying such Software by bioMérieux and by its software publishers. Any other warranty, such as covering performance or results linked with their use, or such as warranty for compatibility with the Buyer's hardware, is expressly excluded. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 14 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Terms & Conditions PAGE 1 OF 5 PAGE 2 OF 5 PAGE 3 OF 5 PAGE 4 OF 5 PAGE 5 OF 5 11. WARRANTIES (CONTINUED) 11.3 Software (continued): THE WARRANTY OF bioMérieux SET FORTH ABOVE AND THE OBLIGATIONS AND bioMérieux will evaluate warranty claims upon receipt of written notification. LIABILITIES OF bioMérieux THEREUNDER ARE EXCLUSIVE AND IN LIEU OF ALL OTHER Firstly, bioMérieux will make a remote diagnosis and if possible, will correct the REMEDIES, WARRANTIES, GUARANTEES OR LIABILITIES, EXPRESSED OR IMPLIED, defect. Secondly, if needed, onsite evaluations will be initiated. Thirdly, if repair ARISING BY LAW OR OTHERWISE, WITH RESPECT TO ANY PRODUCTS DELIVERED cannot be made on the Buyer’s site, the Instrument shall be repaired in HEREUNDER bioMérieux WARRANTY SHALL ONLY APPLY TO PRODUCTS PURCHASED bioMérieux premises. Transportation costs will be at bioMérieux’s expense. The BY THE BUYER FROM bioMérieux. return of the Instrument to bioMérieux can only be done after bioMérieux’s This warranty shall not be extended or altered except by a written agreement signed written approval. by bioMérieux. The Buyer will provide access to bioMérieux, in a timely manner, to any technical support, facilities, hardware, software or information in the Buyer’s possession necessary for bioMérieux to complete such work. bioMérieux will correct such 12. LIABILITY 12.1 bioMérieux accepts no liability for any damage incurred through misuse or stated defects. incorrect storage of Products. In any event, the Buyer is under an obligation to insure that the Instrument As such, Buyer shall ensure the respect of: and/or Software is maintained in accordance with the instructions provided by - bioMérieux such as, but not limited to, written instructions, package insert and user’s manuals and in accordance with local standards during the warranty the storage conditions written on the Products packaging or set forth in the package inserts 12.2 The Buyer or its representative shall take all measures to ensure that period of the concerned Products. instruments, reagents and software are used in compliance with legal Preventive maintenance is not included in the warranty but rather a part of the requirements and standards, including the installation of instruments and imposed maintenance associated with the obligations of the Buyer. performance analysis. As an expert/a man skilled in the art, the Buyer shall use Notwithstanding the foregoing, this warranty will not apply to the extent the the instruments in strict compliance with the recommendations of bioMérieux Instrument and/or Software does not conform to and/or function in accordance and shall remain liable for any interpretation and any use of results provided. with the applicable written technical documentation as a result of a defect The Buyer guarantees bioMérieux against any third party action on the arising from: consequences of using non-compliant Products. - any act or omission of the Buyer - any corporation, joint stock company or any other entity or organization whatsoever, including alleged negligence, shall in no event exceed the purchase of any kind (other than bioMérieux or any person under the express price of the Product in respect of which the claim is made, or at the election of direction of bioMérieux) making any revisions or modifications to the bioMérieux the repair or replacement of such Product plus associated freight Instrument and/or Software after its provision to the Buyer charges. For the avoidance of doubt, bioMérieux’s warranty expressly excludes the malfunction of any Buyer-supplied or Buyer-authorized third-party any possible labour and transport costs involved in the repair of an Instrument. software or equipment IN NO EVENT SHALL bioMérieux BE LIABLE FOR INCIDENTAL OR Buyer operation of the Instrument and/or Software other than in CONSEQUENTIAL DAMAGES WHETHER BUYER'S CLAIM IS IN CONTRACT, accordance with applicable documentation or design, or on hardware not TORT (INCLUDING NEGLIGENCE AND STRICT LIABILITY) OR OTHERWISE. - 12.3 bioMérieux’s liability for any and all losses or damages resulting from any cause recommended, supplied or approved by bioMérieux - the occurrence of any Force Majeure event such as, but not limited to, fire, electrical current variation, flood and defect in the installation environment of the Instrument and/or Software Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 15 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Terms & Conditions PAGE 1 OF 5 PAGE 2 OF 5 PAGE 3 OF 5 PAGE 4 OF 5 13. WASTE MATERIAL Unless anything to the contrary whether expressly agreed by bioMérieux or due to a PAGE 5 OF 5 14. SOFTWARE 14.1 Software included in the price list is subject to a non exclusive license agreement: mandatory local rule, the Buyer is responsible for notifying bioMérieux so that the software programs can neither be reproduced, modified nor transferred without collection, treatment, recovery and environmental sound disposal for the Waste bioMérieux's agreement. Electrical and Electronic Equipment can be organised, as provided for WEEE directive (2002/96/EC) and any local implementations thereof in the country in which the bioMérieux selling company is located. The obligations of the Buyer (in particular in connection with decontamination) to 14.2 Software sold by bioMérieux is free from any known viruses at the date of manufacture. The Buyer shall take all necessary precautions thereafter. 14.3 Moreover, bioMérieux assumes no responsibility for the software compatibility with the hardware of the Buyer. which the Buyer will necessarily have to conform, are defined in the user’s manual of 14.4 On the delivery date, Software supplied by bioMérieux are virus free. bioMérieux the concerned system. The conditions of disposal of these systems, as well as the price interventions are done through tools protected by updated antivirus software. It rates related to these services will be communicated to the Buyer at his request. The is the Buyer‘s responsibility to set up a protection plan for all the data of its Buyer as keeper of the equipment will be responsible for the integrity of the system information system (computing resources of the Buyer) against future risks. and for its disposal at bioMérieux benefit. Every expense incurred or damage 14.5 The Buyer has to make sure of the compatibility of his computer hardware with undergone caused by the Buyer’s negligence and/or any breach of its those proposed by bioMérieux. bioMérieux cannot be held responsible for abovementioned obligations will entitle bioMérieux to a compensation. any contamination by virus. In addition, bioMérieux reserves the right to The Buyer expressly agrees that in case of resale of any Instrument supplied by invoice complementary services due to contamination existing in the Buyer’s bioMérieux and/or transfer of the Instrument in any way, within the framework of a computer hardware. loan or a rent for free or for a fee, or a donation or any other terms, on the national territory or any other territory, then bioMérieux will be released de facto, without any other condition, of its obligations of financing and organization of the elimination of the 15. PERSONAL DATA PROTECTION Personal data of the Buyer (in particular its address and telephone details) collected aforementioned waste material. bioMérieux undertakes to communicate any necessary are subject to processing’s which have been declared to bioMérieux. The Buyer accepts information about the concerned Instruments. The Buyer will be completely that its personal data are used exclusively by bioMérieux and other entities of the responsible for the good documentary, technical and administrative management. The bioMérieux group, in conformity with the provisions of The European Data Protection Buyer will guarantee it towards bioMérieux and will hold bioMérieux harmless for any Directive (95/46/EC). The Buyer has the right to access, modify and rectify such data. damaging consequence, expenses, legal actions from third party including involved For this purpose, the Buyer can contact its account manager. authorities in case of wrongful execution of this obligation. Within the framework of warranty operations of the systems sold by bioMérieux or the quality control of these systems, the Buyer grants access to bioMérieux to the patients personal data for a required period of time in order to perform the abovementioned operation. According to the patient confidentiality regulations applicable in the country in which the bioMérieux selling company is located and The European Data Protection Directive above and after reception of the written instruction of the Buyer, bioMérieux undertakes to set up, from the beginning of the operation, appropriate devices and safety procedures, so as to guarantee strictly the safety; the integrity and the confidentiality of the data pertaining to the Buyer. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 16 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Terms & Conditions PAGE 1 OF 5 PAGE 2 OF 5 PAGE 3 OF 5 PAGE 4 OF 5 PAGE 5 OF 5 16. ASSIGNMENT 16.1 The Buyer shall not assign or transfer the agreement or any of its rights and obligations arising hereunder, or any order covered hereunder, to any other party, including to an Affiliate, without prior written consent of bioMérieux. Any change in the ownership or control of the Buyer shall be deemed an assignment, which requires written consent from bioMérieux. 16.2 The Buyer acknowledges that bioMérieux can transfer the order or be subject to a change of control for the benefit of a third party. 17. APPLICABLE LAW 17.1 These conditions of sales are governed by and construed in accordance with the laws of Sweden, Norway, Denmark and Finland. 17.2 Any dispute shall be settled by the Courts of the country in which the bioMérieux selling company is located, which shall have exclusive jurisdiction, even in case of multiple defendants or claim for contribution from a third party. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 17 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOME Our Principles Sales Orders & Enquiries Customer Support mybiomerieux.com & QC Certificates History bioMérieux Quality & Environment bioMérieux Goes Green Terms & Conditions Legal How to use this Product List Legal This document is not legally binding. bioMérieux reserves the right to modify specifications without notice. bioMérieux, the blue logo, ACCUPROBE, airIDEAL, API, APIWEB, Argene, ATB, BacT/ALERT, bioNexia, Biotools, Cefinase, chromID, Count-Tact, DensiCHEK, DiversiLab, easyMAG, EasyQ, Etest, FIX system, Gonoline, HEPANOSTIKA, Hybridowell, LyfoCults, miniMAG, Mycoline, Myla, NucliSENS, OBSERVA, PolyViteX, PORTAGERM, PREVI, r-gene, R-gene, RAPIDEC, SLIDEX, Toxo-spot-IF, TREPANOSTIKA, UltraClean, URILINE, VIDAS, VIKIA, VIRONOSTIKA and VITEK are registered and protected trademarks belonging to bioMérieux SA or one of its subsidiaries. The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Layout & design © Allso-Print Ltd 2012. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 18 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOW TO USE THIS PRODUCT LIST Overview Layout & Navigation Finding Products Overview In support of the bioMérieux Goes Green initiative, we have produced this interactive PDF Product List. The following notes have been produced with the intention of assisting you to get the best results out of this PDF Product List. As a progressive company, we consider all feedback valuable. If you have any comments or are having problems, please contact us. Our telephone lines are open Monday to Friday (except Bank Holidays) according to the info of office addresses below: Denmark Finland Norway Sweden Order Team Order Team Order Team Order Team Telephone: 70 10 84 02 Telephone: 09 8545 6040 Telephone: 2337 5552 Telephone: 031 68 84 96 Fax: 70 10 84 01 Fax: 09 8545 6045 Fax: 2337 5551 Fax: 031 68 48 48 [email protected] [email protected] [email protected] [email protected] General Enquiries General Enquiries General Enquiries General Enquiries Telephone: 70 10 84 00 Telephone: 09 8545 600 Telephone: 2337 5550 Telephone: 031 68 84 90 Fax: 70 10 84 01 Fax: 09 8545 6045 Fax: 2337 5551 Fax: 031 68 48 48 [email protected] [email protected] [email protected] [email protected] Opening Hours Opening Hours Opening Hours Opening Hours Monday to Thursday Monday to Friday Monday to Friday Monday to Friday from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm from 8.30am to 4.00pm bioMérieux Denmark ApS bioMérieux Suomi Oy bioMérieux Norge AS bioMérieux Sweden AB Smedeholm 13C Konalantie 47C Ulvenveien 75A, 3 etg. Hantverksvägen 15 2730 Herlev 00390 Helsinki 0581 Oslo 43633 Askim Fridays 8.30am to 3.00pm Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 19 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOW TO USE THIS PRODUCT LIST Overview Layout & Navigation Finding Products Layout & Navigation To help you navigate this Product List, every page is separated into these three areas: 2. Tab Menus For every Head Tab, there is a specific Tab Menu shown in the left hand pane. 1 This Tab Menu presents the complete list of contents within the 2 selected Head Tab. 3 If you select a title within the Tab Menu, you advance to the specified page within the Product List. Further Tab Menu options may then be shown if additional pages are available. As with Head Tabs, your selection is highlighted to show your current position. 3. Content The content for the required Head Tab and Tab Menu is shown in the largest portion of the page. The footer bar contains contact details, and will automatically launch e-mail and our web site. 1. Head Tabs There are ten Head Tabs across the top of every page. When the cursor is placed over these Head Tabs, the arrow to a hand changes . By clicking the mouse when the hand icon is over the required Head Tab, you navigate to this section, and the selected tab changes to White to confirm your choice. All of the unselected tabs are shown in Orange. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 20 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY HOW TO USE THIS PRODUCT LIST Overview Layout & Navigation Finding Products Finding Products There are two ways to locate products within this PDF Product List: 1. Using the Head Tabs & Tab Menus 2. Using the Adobe Reader Search Tool You can locate a product by selecting the relevant Head Tab and You can use the Adobe Reader built in search tool to find products by Tab Menu and also by browsing forwards or backwards using name or reference. navigation controls. The search box is usually located in the toolbar above. To open the For instance to find Etest® products, as with previous years these are search box if it is not currently available, choose the ‘Edit’ menu and located in the ‘Identification & Susceptibility Testing’ Tab. Click the then select ‘Find’ or press: ‘Identification & Susceptibility Testing’ Head Tab to select and then click on ‘Etest’ in the Tab Menu. + if you're using a Windows PC + if you're using an Apple Mac Type the word, words or part of the word you want to search for in to the search box. The way in which you cycle through each result depends on the software being used. In most software the first result will be highlighted on the page, to advance to the next result click the arrow next to the search box. This search facility is good for finding product references, as well as every incidence of say, Etest. This makes it the most flexible search tool available within this PDF Product List. Note, Adobe Reader searches from the page currently viewed through to the last page, then continues the search from the front cover until it reaches the page you began on. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 21 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY BacT/ALERT® 3D Culture System The BacT/ALERT 3D provides proven recovery of a wide range of clinically significant organisms, including bacteria, yeasts and mycobacteria. This single system can be used to culture from blood and all normally sterile body fluids using our extensive range of culture media. The same system can be used to culture mycobacteria from decontaminated clinical samples using our Mycobacterial Process bottles or blood using the Mycobacteria Blood bottles. BacT/ALERT 3D System Options The BacT/ALERT 3D system is available in a number of different modules that can be configured to provide a perfect fit no matter what your workload. Larger systems comprise a Controller module with a touch screen for text free control over the basic functions of the system. This unit can be linked to a maximum of six 240 Incubator modules, which would provide sufficient capacity for an annual workload of 84,000 bottles from a single PC. For the laboratory requiring extra space saving the BacT/ALERT 3D combination module combines the computer function and touch screen of a controller module with two incubator drawers. A further three Incubator modules can be linked to this module. Finally the smallest BacT/ALERT 3D 60 can handle an annual workload of up to 3,500 bottles per annum. Configuration The Incubator modules can be configured in either a Stacked or String formation. The String configurations are supplied on purpose built bench carts that have cupboards underneath which are left empty for the department to utilise. This configuration is the most user friendly as all the cells are situated conveniently for the loading and unloading of bottles. The Stacked configuration has the smallest footprint of any blood culture system currently available and is ideal where floor space is at a premium. Simplicity Routine use of the BacT/ALERT 3D system is accessed through the touch screen on Figure 1: String Configuration Figure 2: Stacked Configuration the Controller/Combination Module. The software is intuitive, all tasks are easy to perform and the system is designed to minimise the possibility of users making any mistakes. This ensures that staff can rapidly be trained in day-to-day use. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 22 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY Polycarbonate Blood Culture Bottles Our users also benefit from bioMérieux’s revolutionary, virtually unbreakable, polycarbonate blood culture bottles. These offer enhanced safety as glass is removed from the workplace. The bottles are also significantly lighter than glass ensuring that waste disposal costs are minimal. The bottles are also validated for transport through your pneumatic air system without any need for additional padding or transport containers. This greatly reduces the time taken for these important samples to reach the laboratory. Colorimetric Technology The BacT/ALERT® 3D blood culture system uses the most up to date colorimetric technology. This offers improved time to detection over alternative systems in two ways. First, they provide visual notification of positive bottles as they arrive in the department. This means that bottles may be sub-cultured before they are loaded onto the system thereby improving the turn around time to your clinicians. Negative Bottle Also delayed entry bottles will flag positive two hours after loading. There is no need to wait until these positive bottles enter the death phase of the bacterial life cycle before flagging. Positive Bottle FAN Plus Bottles During 2012 the BacT/ALERT Bottles will also see the world wide launch of the new range of FAN media FA Plus, FN Plus and PF Plus. This media will incorporate Adsorbent Polymer Beads (APB) in place of the activated charcoal to remove antibiotics and naturally occurring bacterial inhibitors, which may be present in the patient’s blood sample. The key benefits are: An increase in overall organism recovery Improved ability to neutralise a broader spectrum of antimicrobials Faster Time to Detection compared to the current bottle types Smaller particles prevent any issues that may be seen with particles blocking sub-culture devices Clear, easy to read Gram stains Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 23 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY Myla™ bioMérieux are proud to introduce you to Myla, an intelligent microbiology middleware solution that is designed by microbiologists for microbiologists. Myla is designed to be accessible from any device linked to the network. This means that it is possible to have blood culture systems sited in satellite hospitals, removing the need to transport bottles between sites and reducing the time taken between blood culture collection and incubation. Staff would be notified to the presence of a positive bottle via a variety of alerting mechanisms. These include standard on screen alerts, optional notification lights, and the ability to send text or e-mail messages. Myla would also alert users to any disruptions in the interface or when error codes are generated on an instrument (e.g. a temperature alert due to a drawer not completely closed on a BacT/ALERT® instrument). With the launch of a new version of Myla software, the functionality with the BacT/ALERT systems was improved. Amongst other features these include a range of workflow indicators: Average time between blood culture order and when the bottle is loaded Average time to positivity Average time between positive detection and bottle unloading for follow up See page 29 for more details of Myla. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 24 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY Next Generation of BacT/ALERT® As part of bioMérieux’s ongoing Research and Development we continue to lead the way with blood culture technology. The next generation of BacT/ALERT will be launched in 2013. The new system will provide many benefits over the existing blood culture systems including automated loading and unloading of blood culture bottles and an even more compact footprint. Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 25 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Blood Culture BacT/ALERT® Computer Systems 413641 411401 NEW OBSERVA® Data Management System Myla™ Middleware One One BacT/ALERT Culture Media 259789 259790 259791 410851 NEW 259793 410852 NEW 259794 410853 NEW BacT/ALERT BacT/ALERT BacT/ALERT BacT/ALERT BacT/ALERT BacT/ALERT BacT/ALERT BacT/ALERT SA Standard Aerobic Culture bottles SN Standard Anaerobic Culture bottles FA FAN Aerobic Culture bottles FA Plus Aerobic Culture bottles FN FAN Anaerobic Culture bottles FN Plus Anaerobic Culture bottles PF Paediatric FAN Culture bottles PF Plus Paediatric Culture bottles 100 100 100 100 100 100 100 100 Bottles Bottles Bottles Bottles Bottles Bottles Bottles Bottles BacT/ALERT Consumables & Accessories 233766 279012 279013 413251 NEW 413201 NEW 413200 NEW 940-0046-01 NEW 259662 206543 BacT/ALERT Airway Sub vent units BacT/ALERT Adaptor caps (blue) BacT/ALERT Adaptor inserts (blue) Blood collector set (21 gauge needle, 30cm tubing) Blood collector set (23 gauge needle, 30cm tubing) Blood collector holder Bottle Transport Rack Generic Barcode Labels Reflectance Standards Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 100 120 60 240 sets 240 sets 180 pieces 48 Wells 2000 1 x 4 Standard www.biomerieux.com 26 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories PREVI™ Fluo TB PREVI Fluo TB Reagents QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Mycobacterial Culture BacT/ALERT® Computer Systems 413641 411401 NEW OBSERVA® Data Management System Myla™ Middleware One One BacT/ALERT Media 259797 259760 251011 259877 BacT/ALERT MP Process Bottle (Polycarbonate) MAS with Vancomycin. Antibiotic supplement BacT/ALERT MB Blood Bottles BacT/ALERT Blood Enrichment Fluid 100 Bottles 5 x 20 25 Bottles 5x5 BacT/ALERT Consumables & Accessories 210361 210362 259787 413251 NEW 413201 NEW 413200 NEW BacT/ALERT Adaptor caps (white) BacT/ALERT Adaptor inserts (white) BacT/ALERT Bottle Reseals Blood collector set (21 gauge needle, 30cm tubing) Blood collector set (23 gauge needle, 30cm tubing) Blood collector holder Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 120 60 100 240 sets 240 sets 180 pieces www.biomerieux.com 27 HOME BLOOD CULTURE / HOW FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION BLOOD CULTURE / MYCOBACTERIAL CULTURE Feature – BacT/ALERT® 3D Culture System & BacT/ALERT 3D System Options Feature – Polycarbonate Blood Culture Bottles, Colorimetric Technology & FAN Plus Bottles Feature – Myla™ Feature – Next Generation of BacT/ALERT Blood Culture BacT/ALERT Computer Systems BacT/ALERT Culture Media BacT/ALERT Consumables & Accessories Mycobacterial Culture BacT/ALERT Computer Systems BacT/ALERT Media BacT/ALERT Consumables & Accessories QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation PREVI™ Fluo TB PREVI Fluo TB Reagents 411010 NEW 411011 NEW 411015 NEW 411014 NEW 411012 NEW 411009 NEW 411013 NEW PREVI Fluo TB kit Fixative Auramine Carbolic Solution Degommier Discolouring Thiazine Red Carbolic Solution for Thiazine Red Up to 200 Tests 1000ml 500ml 500ml 1000ml 500ml 500ml PREVI™ Fluo TB PREVI Fluo TB Reagents Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 28 HOME FULL MICROBIOLOGY HOW BLOOD CULTURE / TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION FULL MICROBIOLOGY LAB AUTOMATION Feature – Full Microbiology Lab Automation & Intelligent Automation Software Myla™ Middleware PREANALYTICAL Urinanalysis Chromogenic Media Direct Identification of E. coli, Proteus & Enterococcus Screening of Multidrug-Resistant Organisms Urinary Bacterial Count QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY FMLA Full Microbiology Lab Automation Full Microbiology Lab Automation focuses on a faster time to result. FMLA brings together bioMérieux’s advances in the automation of operations in Microbiology to deliver greater efficiency, productivity and quality, while retaining the ability to tailor solutions to individual laboratory requirements and enabling the laboratory to effectively respond to increasing workloads. For more details of FMLA, click here. Intelligent Automation PREVI™ Isola PREVI Isola Plate System PREVI Isola Consumables & Accessories PREVI Color PREVI Color Gram System PREVI Color Gram Reagents PREVI Color Gram Consumables & Accessories Our automated solutions offer maximum modularity without compromising result quality and are designed to link to our bespoke intelligent middleware solution, Myla™ to further improve connectivity, data and real-time workflow management. Myla has been designed by microbiologists for microbiologists and plays a central role in the FMLA approach. To find out how this new lab partner helps you simplify your life and recaptures your time, click here. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 29 HOME FULL MICROBIOLOGY HOW BLOOD CULTURE / TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION FULL MICROBIOLOGY LAB AUTOMATION Feature – Full Microbiology Lab Automation & Intelligent Automation Software Myla™ Middleware PREANALYTICAL Urinanalysis Chromogenic Media Direct Identification of E. coli, Proteus & Enterococcus Screening of Multidrug-Resistant Organisms Urinary Bacterial Count PREVI™ Isola PREVI Isola Plate System PREVI Isola Consumables & Accessories QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Software Myla™ Middleware 411401 NEW 411613 NEW 411615 NEW 411616 NEW 411405 NEW 410494 NEW 410493 NEW 410491 NEW 410151 NEW 410152 NEW Myla Middleware Myla VITEK® 2 Connection Kit Myla VITEK MS Connection Kit Myla BacT/ALERT® Connection Kit Myla Monitor LCD 17LN HP L1710 Signal Tower Kit Serial 485 to Ethernet Adapter Cellular Modem Kit Myla Alert / Notification Option Myla Multiuser Licence One 1 Kit 1 Kit Per System 1 Kit 1 Adapter 1 Kit One Licence PREVI Color PREVI Color Gram System PREVI Color Gram Reagents PREVI Color Gram Consumables & Accessories Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 30 HOME FULL MICROBIOLOGY HOW BLOOD CULTURE / TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION FULL MICROBIOLOGY LAB AUTOMATION Feature – Full Microbiology Lab Automation & Intelligent Automation Software Myla™ Middleware PREANALYTICAL Urinanalysis Chromogenic Media Direct Identification of E. coli, Proteus & Enterococcus Screening of Multidrug-Resistant Organisms Urinary Bacterial Count PREVI™ Isola PREVI Isola Plate System PREVI Isola Consumables & Accessories PREVI Color PREVI Color Gram System PREVI Color Gram Reagents PREVI Color Gram Consumables & Accessories QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Urinanalysis Chromogenic Media Direct identification of E. coli, Proteus & Enterococcus 43821 NEW 43829 NEW 43541 43549 43473 411617 NEW 56541 chromID™ CPS chromID CPS chromID CPS chromID CPS chromID CPS/Columbia CNA + 5% sheep blood chromID CPS/Columbia CNA + 5% sheep blood ID indole TDA reagents 20 Plates 100 Plates 20 Plates 100 Plates 20 Plates 20 Plates 2 x 200 Tests Screening of Multidrug-Resistant Organisms 43481 chromID ESBL 20 Plates Urinary Bacterial Count 56507 56508 URILINE™ URILINE Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 10 Dipslides 100 Dipslides www.biomerieux.com 31 HOME FULL MICROBIOLOGY HOW BLOOD CULTURE / TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION FULL MICROBIOLOGY LAB AUTOMATION Feature – Full Microbiology Lab Automation & Intelligent Automation Software Myla™ Middleware PREANALYTICAL Urinanalysis Chromogenic Media Direct Identification of E. coli, Proteus & Enterococcus Screening of Multidrug-Resistant Organisms Urinary Bacterial Count PREVI™ Isola PREVI Isola Plate System PREVI Isola Consumables & Accessories PREVI Color PREVI Color Gram System PREVI Color Gram Reagents PREVI Color Gram Consumables & Accessories QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation PREVI™ Isola PREVI Isola Plate System 29500 PREVI Isola One PREVI Isola Consumables & Accessories 29508 29509 29800 29705 410012 29706 29707 29704 413958 29718 411143 29712 NEW 29713 NEW PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI PREVI Isola Isola Isola Isola Isola Isola Isola Isola Isola Isola Isola Isola Isola tips Applicators Rack 1 Rack 2 Rack 3 UF Rack 4 Rack 5 Rack 6 Swab Rack 10 Enrichment broth Waste Bin Labels Input Cassette Output Cassette Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 50 x 96 tips 8 Cartridges One One One One One One One 10 Waste Bins 1500 Labels One One www.biomerieux.com 32 HOME FULL MICROBIOLOGY HOW BLOOD CULTURE / TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION FULL MICROBIOLOGY LAB AUTOMATION Feature – Full Microbiology Lab Automation & Intelligent Automation Software Myla™ Middleware PREANALYTICAL Urinanalysis Chromogenic Media Direct Identification of E. coli, Proteus & Enterococcus Screening of Multidrug-Resistant Organisms Urinary Bacterial Count PREVI™ Isola PREVI Isola Plate System PREVI Isola Consumables & Accessories PREVI Color PREVI Color Gram System PREVI Color Gram Reagents PREVI Color Gram Consumables & Accessories QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation PREVI™ Color PREVI Color Gram System 29502 29503 29506 PREVI Color Gram 12 PREVI Color Gram 30 PREVI Color Cytocentrifuge Rotor One One One PREVI Color Gram Reagents 29521 29519 29524 29522 29523 29520 29525 Acetone Fuchsin-A Acetone Safranin-A Crystal Violet-C Fuchsin-A Iodine-B Safranin-A Nozzle Cleaning Solution 500ml 500ml 500ml 500ml 500ml 500ml 5 Litres PREVI Color Gram Consumables & Accessories 29559 29558 D&E 500ml Empty Bottle D&E 5L Empty Bottle Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] One One www.biomerieux.com 33 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Qualitative QC Organisms for the Quality Control of API® Identification Strips & VITEK® 2 Cards LyfoCults® Plus are high quality, patented, disposable devices containing lyophilized microorganisms derived from ATCC® cultures. The unique, glass-free, all-in-one device stores and hydrates the freeze-dried microorganisms and has a built-in inoculation wand to streak onto culture media. LyfoCults Plus QC Range ATCC microorganisms packaged in convenient sets for the Quality Control of bioMérieux test kits: VITEK 2 Identification Cards VITEK 2 Susceptibility Cards API Identification Strips ATB™ Susceptibility Strips Individual QC Strains Requirements vary between sites, countries and regulatory authorities but the Clinical and Laboratory Standards Institute (CLSI) recommends that identification products be QC tested once per lot and once per shipment. Click play to view LyfoCults Plus video on YouTube Streamlined QC Sets Recommended for the routine QC testing of API Identification Strips and VITEK 2 Identification Cards Maximum of two organisms chosen to test the least robust and therefore most vulnerable to the effects of shipping, substrate in the Card or Strip One product reference per set for ease of ordering Comprehensive QC Sets Recommended for the validation of a new VITEK 2 system – Performance Qualification Validation of new API Identification Strips or new VITEK 2 Identification Cards Organisms are chosen to reliably test each well or cupule of the Identification Card or Strip for a positive and negative reaction One product reference per set for ease of ordering End User Agreement (EUA) Please Read before ordering from the LyfoCults Plus range. All users of ATCC-derived products must agree to the terms of use by signing the EUA prior to receiving products from bioMérieux The ATCC EUA is a contract that governs the transfer of ATCC-licensed products between two organisations, when the recipient intends to use the licensed products for QC testing EUA protects the rights of all parties to maintain a chain-of-custody from ATCC, to bioMérieux and to the End User, and to assure that the QC microorganisms are traceable to their source It also assures that the product will be used by qualified personnel and handled according to governing laboratory protocols For the LyfoCults Plus End User Agreement, click here. The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 34 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Identification Cards (page 1 of 5) VITEK 2 Gram Negative QC Sets 410586 410601 LyfoCults Plus VITEK 2 GN Streamlined QC set Kit contains: Enterobacter hormaechei Stenotrophomonas maltophilia LyfoCults Plus VITEK 2 GN Comprehensive QC set Kit contains: Acinetobacter baumannii Enterobacter hormaechei Elizabethkingia meningoseptica Klebsiella oxytoca Ochrobactrum anthropi Proteus vulgaris Pseudomonas aeruginosa Pseudomonas aeruginosa Shigella sonnei Stenotrophomonas maltophilia 4 Vials ATCC® 700323™ ATCC 17666™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC BAA-747™ 700323™ 13253™ 700324™ BAA-749™ 6380™ 9721™ BAA-1744™ 25931™ 17666™ 2 Vials 2 Vials 20 Vials 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 35 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Identification Cards (page 2 of 5) VITEK 2 Gram Positive QC Sets 410616 410584 LyfoCults Plus VITEK 2 GP Streamlined QC set Kit contains: Enterococcus casseliflavus Staphylococcus saprophyticus LyfoCults Plus VITEK 2 GP Comprehensive QC set Kit contains: Enterococcus casseliflavus Enterococcus saccharolyticus Kocuria kristinae Listeria monocytogenes Staphylococcus saprophyticus Staphylococcus sciuri ssp. sciuri Streptococcus equi ssp. zooepidemicus Streptococcus pneumoniae Streptococcus thermophilus 4 Vials ATCC® 700327™ ATCC BAA-750™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC 700327™ 43076™ BAA-752™ BAA-751™ BAA-750™ 29061™ 43079™ 49619™ 19258™ 2 Vials 2 Vials 18 Vials 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 36 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Identification Cards (page 3 of 5) VITEK 2 YST QC Sets 410612 410608 LyfoCults Plus VITEK 2 YST Streamlined QC set Kit contains: Candida albicans LyfoCults Plus VITEK 2 YST Comprehensive QC set Kit contains: Candida albicans Candida glabrata Candida lusitaniae Candida utilis Kloeckera japonica Oligella ureolytica Staphylococcus epidermidis Prototheca wickerhamii Sporobolomyces salmonicolor Trichosporon mucoides Zygosaccharomyces bailii 2 Vials ATCC® 14053™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC 14053™ MYA-2950™ 34449™ 9950™ 58370™ 43534™ 12228™ 16529™ MYA-4550™ 204094™ MYA-4549™ 2 Vials 22 Vials 2 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 37 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Identification Cards (page 4 of 5) VITEK 2 NH QC Sets 410628 410611 LyfoCults Plus VITEK 2 NH Streamlined QC set Kit contains: Eikenella corrodens LyfoCults Plus VITEK 2 NH Comprehensive QC set Kit contains: Aggregatibacter aphrophilus Eikenella corrodens Enterobacter aerogenes Haemophilus influenzae Neisseria gonorrhoeae Neisseria lactamica Oligella urethralis Paenibacillus polymyxa Staphylococcus epidermidis 2 Vials ATCC® BAA-1152™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC 33389™ BAA-1152™ 13048™ 9007™ 19424™ 23970™ 17960™ 7070™ 12228™ 2 Vials 18 Vials 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 38 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Identification Cards (page 5 of 5) VITEK 2 ANC QC Sets 410604 410605 LyfoCults Plus VITEK 2 ANC Streamlined QC set Kit contains: Bacteroides ovatus Clostridium septicum LyfoCults Plus VITEK 2 ANC Comprehensive QC set Kit contains: Bacteroides ovatus Bacteroides vulgatus Clostridium perfringens Clostridium septicum Clostridium sordellii Corynebacterium striatum Parabacteroides distasonis 4 Vials ATCC® BAA-1296™ ATCC 12464™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC BAA-1296™ 8482™ 13124™ 12464™ 9714™ BAA-1293™ BAA-1295™ 2 Vials 2 Vials 14 Vials 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 39 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for VITEK® 2 Susceptibility Cards VITEK 2 AST QC Sets 410609 410610 410613 LyfoCults Plus VITEK 2 AST-GN QC set Kit contains: Escherichia coli Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa LyfoCults Plus VITEK 2 AST-GP QC set Kit contains: Enterococcus faecalis Enterococcus faecalis Escherichia coli Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus Streptococcus pneumoniae LyfoCults Plus VITEK 2 AST-YS QC set Kit contains: Candida krusei Candida parapsilosis 8 Vials ATCC® 25922™ ATCC 35218™ ATCC 700603™ ATCC 27853™ ATCC ATCC ATCC ATCC ATCC ATCC ATCC ATCC 29212™ 51299™ 35218™ 29213™ BAA-976™ BAA-977™ BAA-1026™ 49619™ ATCC 6258™ ATCC 22019™ 2 2 2 2 16 Vials Vials Vials Vials Vials 2 2 2 2 2 2 2 2 4 Vials Vials Vials Vials Vials Vials Vials Vials Vials 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 40 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for API® Identification Strips (page 1 of 4) API 20 Strep QC Sets 411002 LyfoCults Plus API 20 Strep Streamlined QC set Kit contains: Streptococcus equi subsp. zooepidemicus LyfoCults Plus API 20 Strep Comprehensive QC set Kit contains: Streptococcus equi subsp. zooepidemicus Streptococcus uberis 410590 2 Vials ATCC® 700400™ 2 Vials 4 Vials ATCC 700400™ ATCC 700407™ 2 Vials 2 Vials API 20 A QC Set 410614 LyfoCults Plus API 20 A QC set Kit contains: Bacteroides ovatus Clostridium perfringens Clostridium sordellii 6 Vials ATCC 8483™ ATCC 13124™ ATCC 9714™ 2 Vials 2 Vials 2 Vials API 20 C AUX QC Sets 411004 410589 LyfoCults Plus API 20 C AUX Streamlined QC set Kit contains: Candida glabrata Cryptococcus laurentii LyfoCults Plus API 20 C AUX Comprehensive QC set Kit contains: Candida glabrata Candida guilliermondii Cryptococcus laurentii 4 Vials ATCC 15126™ ATCC 18803™ 2 Vials 2 Vials 6 Vials ATCC 15126™ ATCC 6260™ ATCC 18803™ 2 Vials 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 41 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for API® Identification Strips (page 2 of 4) API 20 E QC Sets 411003 LyfoCults Plus API 20 E Streamlined QC set Kit contains: Proteus mirabilis LyfoCults Plus API 20 E Comprehensive QC set Kit contains: Enterobacter cloacae Escherichia coli Klebsiella pneumoniae Proteus mirabilis Stenotrophomonas maltophilia 410624 2 Vials ATCC® 35659™ ATCC ATCC ATCC ATCC ATCC 13047™ 25922™ 35657™ 35659™ 51331™ 2 Vials 10 Vials 2 2 2 2 2 Vials Vials Vials Vials Vials API ID 32 GN QC Set 410588 LyfoCults Plus API ID 32 GN QC set Kit contains: Delftia acidovorans Sphingobacterium multivorum 4 Vials ATCC 43868™ ATCC 35656™ 2 Vials 2 Vials API Coryne QC Sets 411001 410625 LyfoCults Plus API Coryne Streamlined QC set Kit contains: Corynebacterium renale LyfoCults Plus API Coryne Comprehensive QC set Kit contains: Cellulosimicrobium cellulans Corynebacterium renale Microbacterium testaceum 2 Vials ATCC 19412™ 2 Vials 6 Vials ATCC 27402™ ATCC 19412™ ATCC 15829™ 2 Vials 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 42 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for API® Identification Strips (page 3 of 4) API 20 NE QC Sets 411006 410626 LyfoCults Plus API 20 NE Streamlined QC set Kit contains: Aeromonas hydrophila Alcaligenes faecalis LyfoCults Plus API 20 NE Comprehensive QC set Kit contains: Aeromonas hydrophila Alcaligenes faecalis Pseudomonas aeruginosa Sphingobacterium multivorum 4 Vials ATCC® 35654™ ATCC 35655™ 2 Vials 2 Vials 8 Vials ATCC ATCC ATCC ATCC 2 2 2 2 35654™ 35655™ 27853™ 35656™ Vials Vials Vials Vials API NH QC Sets 411005 410622 LyfoCults Plus API NH Streamlined QC set Kit contains: Neisseria gonorrhoeae LyfoCults Plus API NH Comprehesive QC set Kit contains: Haemophilus influenzae Aggregatibacter aphrophilus Neisseria gonorrhoeae 2 Vials ATCC 31426™ 2 Vials 6 Vials ATCC 10211™ ATCC 49917™ ATCC 31426™ 2 Vials 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 43 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for API® Identification Strips (page 4 of 4) RapiD 20 E™ QC Set 410621 LyfoCults Plus RapiD 20 E QC set Kit contains: Escherichia coli Klebsiella pneumoniae Proteus hauseri 6 Vials ATCC® 11775™ ATCC 35657™ ATCC 13315™ 2 Vials 2 Vials 2 Vials rapid ID 32 E QC Set 410619 LyfoCults Plus rapid ID 32 E QC set Kit contains: Klebsiella oxytoca Morganella morganii Proteus mirabilis 6 Vials ATCC 43863™ ATCC 25829™ ATCC 35659™ 2 Vials 2 Vials 2 Vials API Staph QC Sets 411000 410620 LyfoCults Plus API Staph Streamlined QC set Kit contains: Staphylococcus capitis LyfoCults Plus API Staph Comprehensive QC set Kit contains: Staphylococcus capitis Staphylococcus lentus Staphylococcus xylosus 2 Vials ATCC 35661™ 2 Vials 6 Vials ATCC 35661™ ATCC 700403™ ATCC 700404™ 2 Vials 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 44 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Reference Description Equivalent to Strain Presentation LyfoCults® Plus QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set 410997 LyfoCults Plus ATB Gram Negative QC set Kit contains: Escherichia coli Escherichia coli Pseudomonas aeruginosa 6 Vials ATCC® 25922™ ATCC 35218™ ATCC 27853™ 2 Vials 2 Vials 2 Vials ATB Gram Positive QC Set 410995 LyfoCults Plus ATB Gram Positive QC set Kit contains: Enterococcus faecalis Streptococcus pneumoniae Staphylococcus aureus Staphylococcus aureus 8 Vials ATCC ATCC ATCC ATCC 29212™ 49619™ 29213™ 43300™ 2 2 2 2 Vials Vials Vials Vials ATB ANA QC Set 410999 LyfoCults Plus ATB ANA QC set Kit contains: Bacteroides fragilis 2 Vials ATCC 25285™ 2 Vials ATB HAEMO QC Set 410996 LyfoCults Plus ATB HAEMO QC set Kit contains: Haemophilus influenzae 2 Vials ATCC 49247™ 2 Vials ATB FUNGUS QC Set 410998 LyfoCults Plus ATB FUNGUS QC set Kit contains: Candida krusei Candida parapsilosis 4 Vials ATCC 6258™ ATCC 22019™ 2 Vials 2 Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 45 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Reference Description Equivalent to Strain Presentation LyfoCults® Plus Individual QC Strains (page 1 of 4) 300772 300750 301215 301192 300765 300776 410270 303485 300803 300836 303489 300826 300850 303524 300862 303592 300868 300784 300805 301497 300905 300908 300927 300921 300914 300953 300947 300945 300959 300962 300965 300983 LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Acinetobacter baumannii Aeromonas hydrophila Aggregatibacter aphrophilus Aggregatibacter aphrophilus Alcaligenes faecalis Aneurinibacillus aneurinolyticus Arcanobacterium haemolyticum Bacillus badius Bacillus circulans Bacillus megabacterium Bacillus pumilus Bacillus subtilis Bacteroides fragilis Bacteroides ovatus Bacteroides ovatus Bacteroides ovatus Bacteroides vulgatus Brevibacilllus agri Brevibacilllus laterosporus Burkolderia cepacia Campylobacter jejuni ssp. jejuni Campylobacter jejuni ssp. jejuni Candida albicans Candida albicans Candida albicans Candida glabrata Candida glabrata Candida guilliermondii Candida krusei Candida lusitaniae Candida parapsilosis Candida utilis ATCC® BAA-747™ ATCC 35654™ ATCC 49917™ ATCC 33389™ ATCC 35655™ ATCC 11376™ ATCC BAA-1784™ ATCC 14574™ ATCC 61™ ATCC 14581™ ATCC BAA-1434™ ATCC 6633™ ATCC 25285™ ATCC BAA-1296™ ATCC 8483™ ATCC BAA-1304™ ATCC 8482™ ATCC 51663™ ATCC 64™ ATCC 25416™ ATCC 33291™ ATCC BAA-1153™ ATCC 14053™ ATCC 10231™ ATCC 90028™ ATCC MYA-2950™ ATCC 15126™ ATCC 6260™ ATCC 6258™ ATCC 34449 ATCC 22019™ ATCC 9950 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 46 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Reference Description Equivalent to Strain Presentation LyfoCults® Plus Individual QC Strains (page 2 of 4) 410279 410282 300996 300986 300993 300999 301000 410276 301016 303528 410273 301039 410291 303600 301064 301153 301053 301072 301067 301105 301090 301095 301107 303502 301145 301131 301140 301122 300873 301182 301201 301198 LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Cellulosimicrobium cellulans Cellulosimicrobium cellulans Cellulosimicrobium cellulans Clostridium difficile Clostridium perfringens Clostridium septicum Clostridium sordellii Corynebacterium renale Corynebacterium renale Corynebacterium striatum Corynebacterium urealyticum Cryptococcus laurentii Curtobacterium pusillum Delftia acidovorans Eikenella corrodens Elizabethkingia meningoseptica Enterobacter aerogenes Enterobacter hormaechei Enterobacter cloacae Enterococcus casseliflavus Enterococcus faecalis Enterococcus faecalis Enterococcus faecalis (VanB) Enterococcus saccharolyticus Escherichia coli Escherichia coli Escherichia coli Escherichia coli Geotrichum capitatum Haemophilus influenzae Haemophilus influenzae Haemophilus influenzae ATCC® BAA-1816™ ATCC BAA-1817™ ATCC 27402 ATCC 9689™ ATCC 13124™ ATCC 12464 ATCC 9714™ ATCC BAA-1785™ ATCC 19412™ ATCC BAA-1293™ ATCC 43044™ ATCC 18803™ ATCC 19096™ ATCC 43868™ ATCC BAA-1152™ ATCC 13253™ ATCC 13048™ ATCC 700323™ ATCC 13047™ ATCC 700327™ ATCC 19433™ ATCC 29212™ ATCC 51299™ ATCC 43076™ ATCC 8739™ ATCC 25922™ ATCC 35218™ ATCC 11775™ ATCC 28576™ ATCC 49247™ ATCC 9007™ ATCC 10211™ 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 47 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Reference Description Equivalent to Strain Presentation LyfoCults® Plus Individual QC Strains (page 3 of 4) 301188 301221 301236 301250 301247 301281 303509 301277 301283 410288 410285 301407 301324 301316 301328 301341 301321 301351 301375 301382 301385 301399 301445 303492 303531 301448 301436 301455 301473 301480 303498 301490 LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Haemophilus influenzae Klebsiella oxytoca Klebsiella oxytoca Klebsiella pneumoniae Klebsiella pneumoniae Kloeckera apiculata var. apis Kloeckera japonica Kocuria kristinae Listeria monocytogenes Microbacterium liquefaciens Microbacterium paraoxydans Microbacterium testaceum Morganella morganii Neisseria gonorrhoeae Neisseria gonorrhoeae Neisseria gonorrhoeae Neisseria gonorrhoeae Neisseria lactamica Ochrobactrum anthropi Oligella ureolytica Oligella urethralis Paenibacillus macerans Paenibacillus polymyxa Paenibacillus validus Parabacteroides distasonis Proteus hauseri Proteus mirabilis Proteus vulgaris Prototheca wickerhamii Pseudomonas aeruginosa Pseudomonas aeruginosa Pseudomonas aeruginosa ATCC® 49766™ ATCC 700324™ ATCC 43863™ ATCC 35657™ ATCC 700603™ ATCC 32857™ ATCC 58370™ ATCC BAA-752™ ATCC BAA-751™ ATCC BAA -1819™ ATCC BAA-1818™ ATCC 15829™ ATCC 25829™ ATCC 19424™ ATCC 43069™ ATCC 49226™ ATCC 31426™ ATCC 23970™ ATCC BAA-749™ ATCC 43534™ ATCC 17960™ ATCC 8509™ ATCC 7070™ ATCC 29948™ ATCC BAA-1295™ ATCC 13315™ ATCC 35659™ ATCC 6380™ ATCC 16529™ ATCC 27853™ ATCC BAA-1744™ ATCC 9721™ 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 48 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY QC ORGANISMS Feature – LyfoCults® Plus LyfoCults Plus QC for VITEK® 2 Identification Cards VITEK 2 Gram Negative QC Sets VITEK 2 Gram Positive QC Sets VITEK 2 YST QC Sets VITEK 2 NH QC Sets VITEK 2 ANC QC Sets QC for VITEK 2 Susceptibility Cards VITEK 2 AST QC Sets QC for API® Identification Strips API 20 Strep QC Sets API 20 A QC Set API 20 C AUX QC Sets API 20 E QC Sets API ID 32 GN QC Set API Coryne QC Sets API 20 NE QC Sets API NH QC Sets RapiD 20 E™ QC Set rapid ID 32 E QC Set API Staph QC Sets QC for ATB™ Susceptibility Strips ATB Gram Negative QC Set ATB Gram Positive QC Set ATB ANA QC Set ATB HAEMO QC Set ATB FUNGUS QC Set Individual QC Strains Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Reference Description Equivalent to Strain Presentation LyfoCults® Plus Individual QC Strains (page 4 of 4) 301526 301579 301583 303514 301589 301616 301586 301627 301624 301621 301630 301634 301640 301650 301658 301662 301664 301697 301668 301701 301707 301728 301731 301744 301762 301757 301767 301773 301780 301783 303518 LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults LyfoCults Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Plus Salmonella typhimurium Shigella sonnei Sphingobacterium multivorum Sporobolomyces salmonicolor Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus Staphylococcus aureus (MRSA) Staphylococcus capitis Staphylococcus epidermidis Staphylococcus epidermidis Staphylococcus lentus Staphylococcus saprophyticus Staphylococcus sciuri ssp. sciuri Staphylococcus xylosus Stenotrophomonas maltophilia Stenotrophomonas maltophilia Streptococcus equi subsp. Zooepidemicus Streptococcus equi subsp. Zooepidemicus Streptococcus pneumoniae Streptococcus pneumoniae Streptococcus pyogenes Streptococcus sp Streptococcus thermophilus Streptococcus uberis Trichophyton mentagrophytes Trichosporon mucoides Virgibacilus pantothenticus Zygosaccharomyces bailii ATCC® 14028™ ATCC 25931™ ATCC 35656™ ATCC MYA-4550™ ATCC 29213™ ATCC BAA-1026™ ATCC 25923™ ATCC BAA-977™ ATCC BAA-976™ ATCC 43300™ ATCC 35661™ ATCC 12228™ ATCC 14990™ ATCC 700403™ ATCC BAA-750™ ATCC 29061™ ATCC 700404™ ATCC 17666™ ATCC 51331™ ATCC 700400™ ATCC 43079™ ATCC 49619™ ATCC 6303™ ATCC 19615™ ATCC 12392™ ATCC 19258™ ATCC 700407™ ATCC 9533™ ATCC 204094™ ATCC 14576™ ATCC MYA-4549™ 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 2 Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials Vials The ATCC Licensed Derivative Emblem, the ATCC Licensed Derivative word mark, and the ATCC catalog marks are trademarks of ATCC. bioMérieux is licensed to use these trademarks and to sell products derived from ATCC cultures. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 49 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides All Purpose Transport Medium ESwab Collection & Transport Medium Specific Transport Medium Urinary Bacterial Count Transport & Culture of Gonococci Transport & Culture of Fungi Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Transport Media & Dipslides All Purpose Transport Medium 42105 41998 41999 41995 PORTAGERM™ agar (tube without swab) PORTAGERM Amies agar & swab - sterile zone PORTAGERM Amies agar + swab PORTAGERM vial for liquid samples 20 Tubes Pack of 10 Units Pack of 50 Units Pack of 10 Vials ESwab Collection & Transport Medium 41992 41990 41991 ESwab - nose, throat, vagina, rectum, wound ESwab - eye, ear, nasal passages, throat, urogenital tract ESwab - naso pharynx and pediatric samples Pack of 50 Units Pack of 50 Units Pack of 50 Units Specific Transport Medium 56528 56525 42041 Gonoline® DUO 2 + CO2 generators Mycoline® PORTAGERM pylori 10 Dipslides 10 Dipslides 8 Bottles x 2.5ml Urinary Bacterial Count 56507 56508 URILINE™ URILINE 10 Dipslides 100 Dipslides Transport & Culture of Gonococci 56528 Gonoline DUO 2 + CO2 generators 10 Dipslides Transport & Culture of Fungi 56525 Mycoline Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 10 Dipslides www.biomerieux.com 50 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media Direct identification of E. coli, Proteus & Enterococcus Direct identification of Candida albicans Detection of Salmonellas Isolation & identification of Staphylococci Screening of S. agalactiae Screening of Multidrug-Resistant Organisms Direct identification of P. aeruginosa Direct identification of Vibrio sp General Purpose Media Specific Media Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Chromogenic Media (page 1 of 2) Direct identification of E. coli, Proteus & Enterococcus 43821 NEW 43829 NEW 43541 43549 43473 411617 NEW 56541 chromID™ CPS chromID CPS chromID CPS chromID CPS chromID CPS/Columbia CNA + 5% sheep blood chromID CPS/Columbia CNA + 5% sheep blood ID indole TDA reagents 20 Plates 100 Plates 20 Plates 100 Plates 20 Plates 20 Plates 2 x 200 Tests Direct identification of Candida albicans 43631 43639 43464 chromID Candida chromID Candida chromID Candida/Sabouraud Gentamicin Chloramphenicol 2 20 Plates 100 Plates 20 Plates Detection of Salmonellas 43621 43465 chromID Salmonella chromID Salmonella/Hektoen 20 Plates 20 Plates Isolation & identification of Staphylococci 43371 43466 NEW chromID S. aureus chromID MRSA/chromID S. aureus Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 20 Plates 20 Plates www.biomerieux.com 51 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media Direct identification of E. coli, Proteus & Enterococcus Direct identification of Candida albicans Detection of Salmonellas Isolation & identification of Staphylococci Screening of S. agalactiae Screening of Multidrug-Resistant Organisms Direct identification of P. aeruginosa Direct identification of Vibrio sp General Purpose Media Specific Media Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Chromogenic Media (page 2 of 2) Screening of S. agalactiae 43461 chromID™ Strepto B 20 Plates Screening of Multidrug-Resistant Organisms 43871 NEW 43861 NEW 43481 43451 43459 43466 NEW 43002 43004 NEW chromID chromID chromID chromID chromID chromID chromID chromID C. difficile CARBA agar ESBL MRSA MRSA MRSA/chromID S. aureus VRE VRE 20 20 20 20 100 20 20 20 Plates Plates Plates Plates Plates Plates Plates Plates Direct identification of P. aeruginosa 43462 chromID P. aeruginosa 20 Plates Direct identification of Vibrio sp 43761 43762 NEW chromID Vibrio chromID Vibrio CE Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 20 Plates 20 Plates www.biomerieux.com 52 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Non Selective agar Non Selective blood agar Selective blood agar Lactose agar Specific Media Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates General Purpose Media (page 1 of 2) Non Selective agar 33606 43011 Nutrient agar Trypcase Soy agar 20 Plates 20 Plates Non Selective blood agar 33633 44282 44117 43101 43109 43050 43059 44813 44724 43041 43049 43401 43061 43001 44279 Blood agar (horse blood) Brain Heart Infusion agar Columbia agar chocolated Chocolate agar + PolyViteX™ Chocolate agar + PolyViteX Columbia agar + 5% horse blood Columbia agar + 5% horse blood Columbia agar + 5% horse blood + NAD Columbia agar + 5% horse blood + NAD Columbia agar + 5% sheep blood Columbia agar + 5% sheep blood Schaedler agar + 5% sheep blood Trypcase Soy agar + 5% horse blood * Trypcase Soy agar + 5% sheep blood Yeast Extract agar 20 20 20 20 100 20 100 20 100 20 100 20 20 20 20 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Columbia agar + Nalidixic Acid + 5% horse blood Columbia CNA agar + 5% sheep blood Columbia CNA agar + 5% sheep blood Schaedler Neomycin Vancomycin agar + 5% sheep blood 20 20 100 10 Plates Plates Plates Plates Selective blood agar 44196 43071 43079 43223 * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 53 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Non Selective agar Non Selective blood agar Selective blood agar Lactose agar Specific Media Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates General Purpose Media (page 2 of 2) Lactose agar 43331 43339 44113 43341 43231 43081 44153 43141 44309 33648 43021 CLED medium CLED medium CLED medium (with Andrade Indicator) Drigalski agar * Endo agar * Eosin Methylene Blue agar MacConkey agar (with salt) MacConkey agar (with salt and crystal violet) MacConkey agar No.2 * MacConkey agar without salt Purple Lactose agar * 20 100 20 20 20 20 20 20 20 20 20 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 54 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Actinomyces Anaerobes Bacillus cereus Bordetella Campylobacter Clostridium difficile Corynebacterium Dermatophytes E. coli O157 Enterobacteriaceae Enterococcus Gardnerella Haemophilus Helicobacter pylori Legionella Listeria Urogenital Mycoplasmas Neisseria Pseudomonas Salmonella, Shigella Staphylococcus Streptococcus Vibrio Yersinia Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Specific Media (page 1 of 5) Actinomyces 44814 Actinomyces agar 10 Plates Anaerobe agar + 5% horse blood Anaerobe agar + Nalidixic acid + Tween + 5% horse blood Anaerobe agar + Neomycin + 5% horse blood Columbia agar + Nalidixic Acid + 5% horse blood Schaedler agar + 5% sheep blood Schaedler Neomycin Vancomycin agar + 5% sheep blood * 20 20 20 20 20 10 Bacillus cereus selective agar 20 Plates Bordet Gengou agar Charcoal agar + Cephalexin 10 Plates 10 Plates Anaerobes 44100 44102 33624 44196 43401 43223 Plates Plates Plates Plates Plates Plates Bacillus cereus 44105 Bordetella 44110 44358 Campylobacter 33627 43361 43369 Campylobacter Selective agar (CCDA) Campylosel agar Campylosel agar 20 Plates 20 Plates 100 Plates Clostridium difficile 43431 Clostridium difficile agar 20 Plates Hoyle medium 10 Plates Corynebacterium 44136 * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 55 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Actinomyces Anaerobes Bacillus cereus Bordetella Campylobacter Clostridium difficile Corynebacterium Dermatophytes E. coli O157 Enterobacteriaceae Enterococcus Gardnerella Haemophilus Helicobacter pylori Legionella Listeria Urogenital Mycoplasmas Neisseria Pseudomonas Salmonella, Shigella Staphylococcus Streptococcus Vibrio Yersinia Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Specific Media (page 2 of 5) Dermatophytes 43464 44815 44125 56525 44176 44175 43651 43659 chromID™ Candida/Sabouraud Gentamicin Chloramphenicol 2 Dermatophyte Test medium Dermatophyte Test medium + Phenol Red Mycoline® Sabouraud Dextrose + Chloramphenicol + Actidione Sabouraud Dextrose agar (with Chloramphenicol) Sabouraud Gentamicin Chloramphenicol 2 agar * Sabouraud Gentamicin Chloramphenicol 2 agar 20 Plates 10 Plates 10 Plates 10 Dipslides 20 Plates 20 Plates 20 Plates 100 Plates E. coli O157 43391 44154 CT-SMAC agar MacConkey agar with Sorbitol (SMAC) 20 Plates 10 Plates Enterobacteriaceae 43821 NEW 43829 NEW 43541 43549 43481 43331 43339 44113 43341 43231 43239 43081 44811 44153 43141 43149 33648 43021 44827 chromID CPS chromID CPS chromID CPS chromID CPS chromID ESBL CLED medium CLED medium CLED medium (with Andrade Indicator) Drigalski agar * Endo agar * Endo agar * Eosin Methylene Blue agar * Membrane Lauryl Sulphate agar MacConkey agar (with salt) MacConkey agar (with salt and crystal violet) MacConkey agar (with salt and crystal violet) MacConkey agar without salt Purple Lactose agar * Tryptone Bile X 20 100 20 100 20 20 100 20 20 20 100 20 20 20 20 100 20 20 20 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 56 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Actinomyces Anaerobes Bacillus cereus Bordetella Campylobacter Clostridium difficile Corynebacterium Dermatophytes E. coli O157 Enterobacteriaceae Enterococcus Gardnerella Haemophilus Helicobacter pylori Legionella Listeria Urogenital Mycoplasmas Neisseria Pseudomonas Salmonella, Shigella Staphylococcus Streptococcus Vibrio Yersinia Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Specific Media (page 3 of 5) Enterococcus 43002 43004 NEW 43151 chromID™ VRE chromID VRE D-Coccosel (Bile Aesculin) agar 20 Plates 20 Plates 20 Plates Gardnerella agar (human blood) 20 Plates Gardnerella 43411 Haemophilus 43101 43681 43689 44813 44117 Chocolate agar + PolyViteX™ Chocolate Haemophilus agar 2 Chocolate Haemophilus agar 2 Columbia agar + 5% horse blood + NAD Columbia agar chocolated 20 20 100 20 20 Plates Plates Plates Plates Plates Helicobacter pylori 43263 Pylori agar 10 Plates Legionella 44143 43013 43023 44146 43031 43032 Legionella Legionella Legionella Legionella Legionella Legionella agar (BCYE) * BCYE with L-cysteine BCYE without L-cysteine Selective agar (MWY) * GVPC medium GVPC medium 20 10 10 20 20 100 Plates Plates Plates Plates Plates Plates Listeria 43560 43559 Listeria Selective agar (Oxford) Palcam agar * 20 Plates 20 Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 57 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Actinomyces Anaerobes Bacillus cereus Bordetella Campylobacter Clostridium difficile Corynebacterium Dermatophytes E. coli O157 Enterobacteriaceae Enterococcus Gardnerella Haemophilus Helicobacter pylori Legionella Listeria Urogenital Mycoplasmas Neisseria Pseudomonas Salmonella, Shigella Staphylococcus Streptococcus Vibrio Yersinia Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Specific Media (page 4 of 5) Urogenital Mycoplasmas 43003 A7 agar * 10 Plates Chocolate agar + PolyViteX™ Chocolate agar + PolyViteX + VCAT 3 New York City medium + LCAT New York City medium + VCAT New York City medium + VCT 20 20 20 20 20 Plates Plates Plates Plates Plates Cepacia Selective agar Cetrimide agar * chromID™ P. aeruginosa Pseudomonas Selective agar (C-F-C) Pseudomonas Selective agar (C-N) 10 20 20 20 20 Plates Plates Plates Plates Plates 20 20 100 20 20 20 20 20 20 100 20 100 20 100 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Neisseria 43101 43611 33625 44163 44824 Pseudomonas 44347 43565 43462 44170 44171 Salmonella, Shigella 43588 43621 43629 43465 44127 44128 44124 43231 43111 43119 43091 43099 43563 43564 Brilliant Green agar modified chromID Salmonella chromID Salmonella * chromID Salmonella/Hektoen DCA agar DCA Hynes agar DCLS Endo agar * Hektoen agar Hektoen agar Salmonella Shigella agar * Salmonella Shigella agar * XLD agar XLD agar * * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 58 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Actinomyces Anaerobes Bacillus cereus Bordetella Campylobacter Clostridium difficile Corynebacterium Dermatophytes E. coli O157 Enterobacteriaceae Enterococcus Gardnerella Haemophilus Helicobacter pylori Legionella Listeria Urogenital Mycoplasmas Neisseria Pseudomonas Salmonella, Shigella Staphylococcus Streptococcus Vibrio Yersinia Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Specific Media (page 5 of 5) Staphylococcus 43521 43451 43459 43466 NEW 43371 43379 44122 44123 43671 43679 44165 Baird-Parker agar chromID™ MRSA chromID MRSA chromID MRSA/chromID S. aureus chromID S. aureus chromID S. aureus DNase agar DNase agar (with Methyl Green) * Mannitol Salt 2 agar Mannitol Salt 2 agar Nutrient agar with 5% salt * 20 20 100 20 20 100 10 10 20 100 20 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates chromID Strepto B Columbia CNA agar + 5% sheep blood G.B.S (Islam) medium * Granada agar Granada/Columbia CNA agar + 5% sheep blood Streptococcus Selective agar (COBA) 20 20 10 20 20 10 Plates Plates Plates Plates Plates Plates chromID Vibrio T.C.B.S. medium 20 Plates 10 Plates Streptococcus 43461 43071 44135 43712 43467 44177 Vibrio 43761 44178 Yersinia 43421 43209 Yersinia CIN Yersinia CIN 20 Plates 100 Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 59 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Yeasts & Moulds Non Selective agar Selective agar Media for Antibiotic Susceptibility: Disc Diffusion Methods Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Yeasts & Moulds Non Selective agar 44156 44715 44174 Malt Extract agar * Malt Extract irradiated agar Sabouraud Dextrose agar 20 Plates 20 Contact Plates 20 Plates chromID™ Candida chromID Candida/Sabouraud Gentamicin Chloramphenicol 2 Dermatophyte Test medium Dermatophyte Test medium + Phenol Red Mycoline® Sabouraud Dextrose agar Sabouraud Dextrose irradiated agar Sabouraud Dextrose agar (with Chloramphenicol) Sabouraud Dextrose Chloramphenicol agar Sabouraud Dextrose + Chloramphenicol + Actidione agar Sabouraud Gentamicin Chloramphenicol 2 agar * 20 Plates 20 Plates 10 Plates 10 Plates 10 Dipslides 20 Plates 20 Contact Plates 20 Plates 20 Plates 20 Plates 20 Plates Selective agar 43631 43464 NEW 44815 44125 56525 43555 NEW 44717 44175 43596 NEW 44176 43651 * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 60 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION CULTURE MEDIA QC ORGANISMS IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Chromogenic Media General Purpose Media Specific Media Yeasts & Moulds Media for Antibiotic Susceptibility: Disc Diffusion Methods BSAC compliant media NCCLS compliant media Divided Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates Media for Antibiotic Susceptibility: Disc Diffusion Methods BSAC compliant media 44321 33620 44138 44198 44721 Columbia agar + 2% NaCl Sensitivity Test agar Sensitivity Test agar + 5% horse blood Sensitivity Test agar + 5% horse blood + NAD Sensitivity Test agar + 5% horse blood + NAD 10 20 20 10 100 Plates Plates Plates Plates Plates 20 20 20 Square 20 100 20 20 Plates Plates Plates Plates Plates Plates Plates NCCLS compliant media 33612 43302 43511 43301 43309 43321 43901 Haemophilus Test medium (HTM) * Mueller Hinton 2 agar (150mm) * Mueller Hinton 2 agar (120 x 120mm) * Mueller Hinton 2 agar * Mueller Hinton 2 agar * Mueller Hinton 2 agar + 5% sheep blood * Mueller Hinton agar + 5% horse blood agar + NAD Divided Plates 44807 43464 43473 43466 NEW 43465 41167 43467 44808 Anaerobe agar + Neomycin + 5% horse blood/ Columbia + 5% horse blood chromID™ Candida/Sabouraud Gentamicin Chloramphenicol 2 chromID CPS/Columbia CNA + 5% sheep blood chromID MRSA/chromID S. aureus chromID Salmonella/Hektoen chromID4 CPS/Columbia CNA + 5% sheep blood Granada/Columbia CNA agar + 5% sheep blood New York City medium + LCAT/Sabouraud agar + Chloramphenicol 20 Plates 20 20 20 20 20 20 20 Plates Plates Plates Plates Plates Plates Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 61 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications Irradiated media Page 1 of 2 Page 2 of 2 Sterility Testing media Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications (page 1 of 3) Irradiated media (page 1 of 2) 96301 43501 NEW 43491 43492 43593 43691 NEW 43699 NEW 96300 43580 NEW 43581 43812 NEW 43582 NEW 44229 44300 44715 44717 44343 44301 43554 43814 NEW 43595 Bi-Box (double wrapped and irradiated) † Count-Tact™ agar Count-Tact irradiated agar † Count-Tact irradiated agar † Count-Tact irradiated agar (isolator) † Count-Tact 3P™ irradiated agar † Count-Tact 3P irradiated agar † Count-Tact Applicator † Count-Tact Sabouraud Dextrose agar with Chloramphenicol and neutralisers † Count-Tact Sabouraud Dextrose irradiated agar with Chloramphenicol and neutralisers † Count-Tact Sabouraud Dextrose 3P irradiated agar with neutralizers † Count-Tact Trypcase Soy agar † Environmental Monitoring irradiated agar * Environmental Monitoring irradiated agar isolator * Malt Extract irradiated agar Sabouraud Dextrose irradiated agar Sabouraud Dextrose irradiated agar (140mm) * Sabouraud Dextrose irradiated agar isolator * Sabouraud Dextrose 3P irradiated agar Sabouraud Dextrose 3P irradiated agar with neutralizers Sabouraud Dextrose Chloramphenicol irradiated agar 10 x 10 Contact Plate Boxes 20 Contact Plates 20 Contact Plates 100 Contact Plates 20 Contact Plates 20 Contact Plates 100 Contact Plates One 20 Contact Plates 20 Contact Plates 20 Contact Plates 20 Contact Plates 20 Plates 20 Plates 20 Contact Plates 20 Contact Plates 4 x 5 Plates 20 Plates 20 Plates 20 Plates 20 Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. † FIX system™ Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 62 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications Irradiated media Page 1 of 2 Page 2 of 2 Sterility Testing media Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications (page 2 of 3) Irradiated media (page 2 of 2) 43557 D1113 43131 43556 43594 43562 D1106 43711 NEW 43169 43811 NEW 43819 NEW Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Trypcase Soy Soy Soy Soy Soy Soy Soy Soy Soy Soy Soy irradiated agar irradiated agar † irradiated agar † irradiated agar (140mm) irradiated agar (isolator) irradiated agar with neutralisers irradiated agar with Penicillinase * † 3P™ irradiated agar 3P irradiated agar 3P irradiated agar with neutralizers 3P irradiated agar with neutralizers 100 100 Contact 20 20 20 100 20 Contact 20 100 20 100 Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. † FIX system™ Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 63 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications Irradiated media Sterility Testing media Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Plates, Tubes & Bottles Pharmaceutical Applications (page 3 of 3) Sterility Testing media 43558 NEW 42616 42044 44502 42074 44001 44277 42100 41146 44288 44011 44362 Plate Count agar Rinsing Solution irradiated Rinsing Solution (EP, USP fluid A) irradiated Thioglycollate broth Thioglycollate broth + resazurin Thioglycollate USP with injectable cap Trypcase Soy broth Trypcase Soy broth Trypcase Soy broth Trypcase Soy broth Trypcase Soy broth (EP, USP) irradiated Trypcase Soy broth (wnb) * 20 Plates 4 x 300ml 12 x 100ml 10 x 110ml 20 Tubes x 9ml 12 x 100ml 10 x 50ml 20 Tubes x 9ml 6 x 100ml 50 x 20ml 12 x 100ml 10 x 90ml * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. wnb – wide necked bottle. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 64 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media E. coli & coliforms General Purpose Media Non Selective broth Non Selective agar Diluent Specific Media Yeasts & Moulds Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Tubes & Bottles Chromogenic Media E. coli & coliforms 42605 42017 chromID™ O157:H7 Coli ID 6 x 200ml 6 x 200ml General Purpose Media (page 1 of 2) Non Selective broth 44260 42081 44327 44271 42100 44266 41146 44288 Brain Heart Infusion broth Brain Heart Infusion broth Nutrient broth * Nutrient broth * Trypcase Soy broth Trypcase Soy broth Trypcase Soy broth Trypcase Soy broth 50 x 9ml 20 Tubes x 9ml 50 x 5ml 50 x 9ml 20 Tubes x 9ml 50 x 9ml 6 x 100ml 50 x 20ml Non Selective agar 41536 44333 42079 41244 44283 43551 NEW 42101 44267 43019 NEW 41467 41466 Chocolate agar Chocolate agar (slopes) Chocolate agar + PolyViteX™ (slopes) Columbia agar Nutrient agar (slopes) R2A agar Trypcase Soy agar (slopes) Trypcase Soy agar (slopes) Trypcase Soy agar Trypcase Soy agar Trypcase Soy agar 6 x 100ml 50 x 3.5ml 20 Tubes 6 x 200ml 50 x 5ml 20 Plates 20 Tubes 50 x 15ml 100 Plates 6 x 100ml 6 x 200ml * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 65 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media E. coli & coliforms General Purpose Media Non Selective broth Non Selective agar Diluent Specific Media Yeasts & Moulds Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Reference Description Presentation Pre-Poured Media – Tubes & Bottles General Purpose Media (page 2 of 2) Diluent 42076 NEW 42085 44286 44287 44332 44329 Maximum Recovery Diluent Peptone water Peptone water Purified water Saline Solution 0.85% Tryptone water (Report 71) * 20 Tubes x 9ml 20 Tubes x 9ml 50 x 3ml 50 x 4.5ml 50 x 3ml 50 x 5ml * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 66 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media General Purpose Media Specific Media Anaerobes Corynebacterium Enterobacteriaceae Enterococcus Legionella Listeria Mycobacteria Mycoplasma hominis, Ureaplasma urealyticum Salmonella, Shigella Staphylococcus Trichomonas Vibrio Streptococci Yeasts & Moulds Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Tubes & Bottles Specific Media Anaerobes 44272 41936 42106 42097 42098 42074 Cooked Meat medium Schaedler agar (+ Vit. K3) Schaedler broth (+ Vit. K3) Schaedler broth + 0.02% agar (+ Vit. K3) Schaedler broth + 0.2% agar (+ Vit. K3) Thioglycollate broth + resazurin 50 x 15ml 6 x 100ml 20 Tubes x 13ml 20 Tubes x 13ml 20 Tubes x 13ml 20 Tubes x 9ml Corynebacterium 42088 Loeffler medium (slopes) 20 Tubes CLED agar Eosin Methylene Blue agar 6 x 200ml 6 x 100ml Enterobacteriaceae 41594 41336 Enterococcus 44334 42083 Aesculin broth * D-Coccosel (Bile Aesculin) agar 50 x 3ml 20 Tubes Legionella 41054 55641 55645 Legionella - BCYE agar base Legionella enrichment supplement Legionella selective mixture 6 x 200ml 4 x 2ml 4 x 2ml Buffered Listeria Enrichment broth Fraser broth Fraser broth Fraser broth (half) Listeria broth (UVM I) 10 x 225ml 20 Tubes x 10ml 6 x 225ml 6 x 225ml 50 x 10ml Listeria 44302 42072 42046 42048 44523 * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 67 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media General Purpose Media Specific Media Anaerobes Corynebacterium Enterobacteriaceae Enterococcus Legionella Listeria Mycobacteria Mycoplasma hominis, Ureaplasma urealyticum Salmonella, Shigella Staphylococcus Trichomonas Vibrio Streptococci Yeasts & Moulds Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Tubes & Bottles Specific Media Mycobacteria 42082 42107 42089 Coletsos medium (slopes) Lowenstein-Jensen + TCH (slopes) Lowenstein-Jensen medium (slopes) 20 Tubes 20 Tubes 20 Tubes Mycoplasma hominis, Ureaplasma urealyticum 42508 Urea-arginine LYO-2 broth 25 Tests Salmonella, Shigella 42042 42043 41554 44536 42114 NEW 42091 42099 44263 Buffered Peptone water Buffered Peptone water Hektoen agar Mannitol Selenite broth Muller Kauffmann Tetrathionate and novobiocin broth (MKTTn) Rappaport broth Selenite-F broth Selenite-F broth 6 x 90ml 6 x 225ml 6 x 200ml 50 x 15ml 20 x 10ml 20 Tubes x 9ml 20 Tubes x 9ml 50 x 15ml Staphylococcus 44003 44259 42080 Baird Parker & RPF agar Brain Heart Infusion broth + 7% salt * Mannitol Salt agar (slopes) 4 x 100ml 50 x 9ml 20 Tubes Diamonds medium 50 x 4.5ml Alkaline Peptone water 50 x 10ml Trichomonas 44526 Vibrio 44261 Streptococci 42116 NEW Todd Hewitt broth selective enrichment for detection of group B streptococci in pregnant women 20 Tubes x 9ml * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 68 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media General Purpose Media Specific Media Yeasts & Moulds Non Selective Media Selective Media Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Tubes & Bottles Yeasts & Moulds Non Selective Media 44524 42037 42066 44253 42108 Cornmeal agar * Sabouraud 2 agar Sabouraud 2 agar Sabouraud Dextrose agar Sabouraud liquid broth 50 x 20ml 20 Tubes 6 x 100ml 10 x 250ml 20 Tubes x 9ml Selective Media 42084 42067 42038 42094 42031 42056 42096 44525 Dermatophyte Test agar (slopes) Sabouraud Chloramphenicol 2 agar Sabouraud Chloramphenicol 2 agar Sabouraud Chloramphenicol Actidione agar (slopes) Sabouraud Gentamicin Chloramphenicol 2 agar Sabouraud Gentamicin Chloramphenicol 2 agar Sabouraud Tetrazolium Genta. Chloramphenicol agar (slopes) Yeast Extract agar 20 Tubes 6 x 100ml 20 Slopes 20 Tubes 20 Tubes 6 x 100ml 20 Tubes 10 x 100ml Media for Biochemical Identification 42086 42087 42085 44284 55752 Esculin agar Kligler iron agar (stab/slope) Peptone water Urea agar Urea Indole medium 20 Tubes 20 Tubes 20 Tubes x 9ml 50 Agar Slopes 10 x 10ml * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 69 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Chromogenic Media General Purpose Media Specific Media Yeasts & Moulds Media for Biochemical Identification Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods Media for Mycobacteria Sputum digestion & decontamination Staining Culture Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Pre-Poured Media – Tubes & Bottles Media for Antibiotic Susceptibility Testing: Disc Diffusion Methods 41864 Mueller Hinton 2 agar 6 x 200ml Media for Mycobacteria Sputum digestion & decontamination 42104 42103 55242 R2 : benzalkonium chloride R3 : Phosphate Buffer R4 : solution of bovine albumin 20 Tubes x 10ml 20 Tubes x 15ml 16 x 10ml Staining 55531 55521 Gabett solution Kinyoun solution 1 x 450ml 1 x 450ml Culture 42082 42107 42089 Coletsos medium (slopes) Lowenstein-Jensen + TCH (slopes) Lowenstein-Jensen medium (slopes) Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 20 Tubes 20 Tubes 20 Tubes www.biomerieux.com 70 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Culture, Identification Enumeration & Susceptibility Testing Enumeration Detection Collection & Culture Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Reference Description Presentation Urogenital Mycoplasmas Culture, Identification Enumeration & Susceptibility Testing Enumeration 42505 Mycoplasma IST 2 25 Tests Urea-arginine LYO-2 broth 25 Tests Detection 42508 Collection & Culture 42507 43003 Mycoplasma Preparation (collection + transport) A7 agar * 8 Tests 10 Plates * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 71 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox GENbag Generators Indicator Accessories GENbox Generators Indicator Air Sampling Dehydrated Media Supplements Reference Description Presentation Incubation System: GENbag-GENbox GENbag Generators 45534 45533 45532 GENbag Anaer GENbag CO2 GENbag Microaer 20 Sachets 20 Sachets 20 Sachets Indicator 96118 GENbag/box Anaer indicator 50 Tests Accessories 45511 GENbag clip seals Pack of 10 GENbox 96127 96128 96129 96130 GENbox GENbox GENbox GENbox 2.5L 7L Lid 2.5L Lid 7L 12 Plate capacity 42 Plate capacity 1 Lid 1 Lid GENbox Anaer GENbox CO2 GENbox Microaer 10 Sachets 10 Sachets 10 Sachets Generators 96124 96126 96125 Indicator 96118 GENbag/box Anaer indicator Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 50 Tests www.biomerieux.com 72 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Supplements Implement a Simple Traceability Solution in Your Facility with airIDEAL® 3P™ Traceability & 3P Plates airIDEAL 3P Traceability has all of the benefits as before, but now incorporates features to allow full traceability of all sampling operations Implement a simple traceability solution or compliment your existing electronic record keeping Complete audit trail that includes technician, sampler, individual agar plate and sample The full traceability package comprises 3 components: airIDEAL 3P Traceability air sampler unit with indicating LED light Remote control with embedded software and built-in 2D-barcode scanner (Datamatrix) 21 CFR Part 11 compliant and network-capable software for designing and following air sampling campaigns ISO 14698 validation for physical and biological efficiency, H2O2 isolator compatibility and air circuit decontamination. Reference Description Presentation Air Sampling 410174 410175 410173 410169 96304 96309 96308 airIDEAL 3P Traceability (air sampler Contact Plates) airIDEAL 3P Traceability (air sampler 90mm Plates) airIDEAL RUID Remote Control airIDEAL Traceability CD Software Sampling Screen (Contact Plates) Sampling Screen (90mm) Tripod Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] Instrument Instrument Instrument Software One One One www.biomerieux.com 73 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Page 1 of 2 Page 2 of 2 Supplements Reference Description Presentation Dehydrated Media 51024 51074 51065 51039 51009 51045 51003 51052 51026 51025 51029 51027 51032 51033 51022 51034 51013 51050 51059 51014 51036 51015 51053 51075 51035 51077 51078 51063 51043 51076 51017 51072 Azide dextrose broth (Rothe) Baird Parker agar base Bismuth Sulphite agar (Wilson Blair) Blood agar base Brain Heart Infusion Brilliant Green agar Brilliant Green bile broth CLED agar Columbia agar D-Coccosel agar - Aesculia Bile agar Desoxycholate citrate agar Desoxycholate lactose agar Endo agar Eosin Methylene Blue agar Ethyl violet azide broth (Litsky) Eugon agar Eugon broth Hektoen agar Kligler iron agar Lactose broth MacConkey agar without crystal violet MacConkey broth Mannitol Salt agar Mueller Hinton 2 agar Purple Lactose agar Sabouraud agar Sabouraud Chloramphenicol agar Sabouraud liquid broth Salmonella Shigella agar Schaedler broth Selenite-F broth Standard methods agar (PCA) Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g 500g www.biomerieux.com 74 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY CULTURE MEDIA Transport Media & Dipslides Pre-Poured Media – Plates Pre-Poured Media – Plates, Tubes & Bottles Pre-Poured Media – Tubes & Bottles Urogenital Mycoplasmas Incubation System: GENbag-GENbox Air Sampling Dehydrated Media Page 1 of 2 Page 2 of 2 Supplements Reference Description Presentation Dehydrated Media 51018 51047 51044 51019 51048 51005 51049 Thioglycollate broth + resazurin Triple sugar iron agar Trypcase Soy agar Trypcase Soy broth TSN agar Violet Red Bile Lactose agar XLD agar 500g 500g 500g 500g 500g 500g 500g Supplements 55691 55673 55682 55641 55645 55651 55652 55501 55663 Clostridium difficile mixture CNA mixture Kanamycin - Vancomycin mixture Legionella enrichment supplement Legionella selective mixture PolyViteX™ mixture PolyViteX mixture Potassium tellurite solution VCN mixture Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 4 x 2ml 8 x 2ml 8 x 1ml (Lyophilised) 4 x 2ml 4 x 2ml 4 x 1ml + Diluent 4 x 10ml + Diluent 6 x 5ml 8 x 1ml www.biomerieux.com 75 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MOLECULAR BIOLOGY MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description Presentation Orientation Tests Staining Catalase, Oxidase Other Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Orientation Tests Staining 55542 55545 55546 55547 55548 55531 55521 Color Gram 2, kit Color Gram 2, R1 Color Gram 2, R2 Color Gram 2, R3 Color Gram 2, R4 Gabett solution Kinyoun solution Oxalate Crystal Violet Lugol PVP Decolorizer Safranin 2 2 2 2 4 x 240ml Litre Bottle Litre Bottle Litre Bottle Litre Bottle 1 x 450ml 1 x 450ml Catalase, Oxidase 55561 55635 54991 ID color Catalase Oxidase Reagent Paper discs (for use with Oxidase Reagent) 2 x 5ml 50 Ampoules x 0.75ml 50 discs Other Tests 50110 55631 55601 55912 55501 55181 55182 API® OF medium Kovacs reagent ONPG Optochin test Potassium tellurite solution Rabbit plasma Rabbit plasma Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 10 Ampoules x 5ml 1 x 25ml 30 discs 2 x 30 discs 6 x 5ml 8 x 0.5ml 1 x 3.5ml www.biomerieux.com 76 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification SLIDEX®: Latex Agglutination Staphylococcus Streptococcus Streptococcus pneumoniae Meningitis RAPIDEC®: Identification in 2 Hours Direct IF: Immunofluoresecence Antibody API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Rapid Antigen Detection & Identification SLIDEX®: Latex Agglutination Staphylococcus 73115 73116 SLIDEX Staph-Plus SLIDEX Staph-Plus 50 Tests 250 Tests (Stirring Sticks not included) Streptococcus 58811 58818 58819 SLIDEX Strepto-Plus SLIDEX Strepto-Plus A SLIDEX Strepto-Plus B 40-50 Tests 40 Tests 40 Tests Streptococcus pneumoniae 58821 SLIDEX pneumo-Kit 50 Tests SLIDEX meningite Strepto B SLIDEX meningite-Kit 5 30 Tests 25 Tests Meningitis 58831 58803 Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 77 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification SLIDEX®: Latex Agglutination RAPIDEC®: Identification in 2 Hours RAPIDEC staph RAPIDEC ur RAPIDEC Listeria monocytogenes Direct IF: Immunofluoresecence Antibody Chlamydia RSV (Respiratory Synctytial Virus) API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Rapid Antigen Detection & Identification RAPIDEC®: Identification in 2 Hours RAPIDEC staph 03300 70500 70700 RAPIDEC staph API® FVB Suspension medium (2ml) 30 Tests 5ml Bottle 100 Ampoules RAPIDEC ur Suspension medium (2ml) API FVB JAMES Reagent 30 Tests 100 Ampoules 5ml Bottle 2 x 5ml Ampoules RAPIDEC ur 03200 70700 70500 70542 RAPIDEC Listeria monocytogenes 03700 RAPIDEC Lmono 20 Tests Direct IF: Immunofluoresecence Antibody Chlamydia 55331 55321 75741 55311 Chlamydia Direct IF prelevement (collection kit) Chlamydia Direct IF identification Direct IF slides Chlamydia Direct IF control +/- 20 Kits 50 Tests 100 Slides 10 Slides RSV (Respiratory Synctytial Virus) 55341 55351 RSV Direct IF identification RSV Direct IF control +/- 50 Tests 10 Slides Rapid Antigen Detection of Clostridium difficile toxin with VIDAS® and mini VIDAS®, see page 110. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 78 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Page 1 of 2 Page 2 of 2 Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer ® QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification Identification Software 40011 40012 APIWEB® APIWEB Stand Alone Licence CD API & RapiD Strips (page 1 of 7) Enterobacteriaceae (page 1 of 2) API 20 E 20100 20120 70442 50110 70402 70422 70380 70542 70100 20230 55635 20150 411003 410624 API 20 E API 20 E reagent kit * Kit contains: JAMES Reagent API NIT 1 & NIT 2 API VP 1 & VP 2 API TDA API NIT 1 & NIT 2 API OF medium API TDA API VP 1 & VP 2 API Zn JAMES Reagent Mineral oil NaCl 0.85% (5ml) Oxidase Reagent Suspension medium (5ml) LyfoCults® Plus API 20 E Streamlined QC set ‡ LyfoCults Plus API 20 E Comprehensive QC set ‡ 25 Strips 6 x 5ml Ampoules (w/o dropper-bottles) 2 x 5ml NIT 1; 2 x 5ml NIT 2 10 Ampoules x 5ml 2 x 5ml Ampoules 2 x 5ml VP 1; 2 x 5ml VP 2 2 x 10g 2 x 5ml Ampoules 125ml Bottle 100 Ampoules 50 Ampoules x 0.75ml 100 Ampoules 2 Vials 10 Vials * Standing orders are recommended. This product may have a 6 week lead time, please contact us to check for availability. ‡ See LyfoCults Plus page 42 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 79 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Page 1 of 2 Page 2 of 2 Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 2 of 7) Enterobacteriaceae (page 2 of 2) API 10 S 10100 70442 70402 70542 70100 20230 55635 20150 API 10 S API NIT 1 & NIT 2 API TDA JAMES Reagent Mineral oil NaCl 0.85% (5ml) Oxidase Reagent Suspension medium (5ml) 50 Strips 2 x 5ml NIT 1; 2 x 5ml NIT 2 2 x 5ml Ampoules 2 x 5ml Ampoules 125ml Bottle 100 Ampoules 50 Ampoules x 0.75ml 100 Ampoules RapiD 20 E™ (Identification in 4 hours) 20701 70422 70542 20070 55635 20710 410621 RapiD 20 E API VP 1 & VP 2 JAMES Reagent NaCl 0.85% medium (2ml) Oxidase Reagent RapiD 20 E Suspension medium LyfoCults® Plus RapiD 20 E QC set ‡ 25 Strips 2 x 5ml VP 1; 2 x 5ml VP 2 2 x 5ml Ampoules 100 Ampoules 50 Ampoules x 0.75ml 25 x 1.25ml 6 Vials API 50 CH 50300 50430 70902 70100 70700 20150 API 50 CH API 50 CHB/E medium Ampoule guards (50 CH range) Mineral oil Suspension medium (2ml) Suspension medium (5ml) 10 Strips 10 Ampoules Pack of 3 125ml Bottle 100 Ampoules 100 Ampoules ‡ See LyfoCults Plus page 44 for strains contained within the set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 80 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 3 of 7) Non Enteric Gram Negative rods API 20 NE 20050 70442 70380 70542 70100 20070 55635 411006 410626 API 20 NE API NIT 1 & NIT 2 API Zn JAMES Reagent Mineral oil NaCl 0.85% medium (2ml) Oxidase Reagent LyfoCults® Plus API 20 NE Streamlined QC set ‡ LyfoCults Plus API 20 NE Comprehensive QC set ‡ 25 Strips & media 2 x 5ml NIT 1; 2 x 5ml NIT 2 2 x 10g 2 x 5ml Ampoules 125ml Bottle 100 Ampoules 50 Ampoules x 0.75ml 4 Vials 8 Vials API Staph API NIT 1 & NIT 2 API VP 1 & VP 2 API ZYM A API ZYM B Mineral oil LyfoCults Plus API Staph Streamlined QC set ‡ LyfoCults Plus API Staph Comprehensive QC set ‡ 25 Strips & media 2 x 5ml NIT 1; 2 x 5ml NIT 2 2 x 5ml VP 1; 2 x 5ml VP 2 2 x 8ml Ampoules 2 x 8ml Ampoules 125ml Bottle 2 Vials 6 Vials Staphylococcus API Staph 20500 70442 70422 70494 70493 70100 411000 410620 ‡ See LyfoCults Plus pages 43 and 44 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 81 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 4 of 7) Streptococcus API 20 Strep 20600 70491 70422 70494 70493 70100 70700 411002 410590 API 20 Strep API NIN API VP 1 & VP 2 API ZYM A API ZYM B Mineral oil Suspension medium (2ml) LyfoCults® Plus API 20 Strep Streamlined QC set ‡ LyfoCults Plus API 20 Strep Comprehensive QC set ‡ 25 Strips & media 2 x 5ml Bottle 2 x 5ml VP 1; 2 x 5ml VP 2 2 x 8ml Ampoules 2 x 8ml Ampoules 125ml Bottle 100 Ampoules 2 Vials 4 Vials Neisseria & Haemophilus API NH 10400 70100 411005 410622 API NH Mineral oil LyfoCults Plus API NH Streamlined QC set ‡ LyfoCults Plus API NH Comprehensive QC set ‡ 10 Strips & media 125ml Bottle 2 Vials 6 Vials Corynebacterium API Coryne 20900 70442 70492 70494 70493 70100 411001 410625 API Coryne API NIT 1 & NIT 2 API PYZ API ZYM A API ZYM B Mineral oil LyfoCults Plus API Coryne Streamlined QC set ‡ LyfoCults Plus API Coryne Comprehensive QC set ‡ 12 Strips & media 2 x 5ml NIT 1; 2 x 5ml NIT 2 2 x 5ml Bottle 2 x 8ml Ampoules 2 x 8ml Ampoules 125ml Bottle 2 Vials 6 Vials ‡ See LyfoCults Plus pages 41 to 43 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 82 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 5 of 7) Campylobacter API Campy 20800 70562 70442 70100 55635 API Campy API FB API NIT 1 & NIT 2 Mineral oil Oxidase Reagent 12 Strips & media 2 x 5ml Ampoules 2 x 5ml NIT 1; 2 x 5ml NIT 2 125ml Bottle 50 Ampoules x 0.75ml Anaerobes API 20 A 20300 70510 70520 70530 70100 410614 API 20 A API BCP API EHR API XYL Mineral oil LyfoCults® Plus API 20 A QC set ‡ 25 Strips & media 5ml Bottle 5ml Bottle 5ml Bottle 125ml Bottle 6 Vials API Listeria 10 Strips & media Listeria API Listeria 10300 ‡ See LyfoCults Plus page 41 for strains contained within the set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 83 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 6 of 7) Bacillus API 50 CH 50300 50430 70902 70100 API 50 CH API 50 CHB/E medium Ampoule guards (50 CH range) Mineral oil 10 Strips 10 Ampoules Pack of 3 125ml Bottle API 50 CH API 50 CHL medium Ampoule guards (50 CH range) Mineral oil Suspension medium (2ml) Suspension medium (5ml) 10 Strips 10 Ampoules Pack of 3 125ml Bottle 100 Ampoules 100 Ampoules Lactobacillus API 50 CH 50300 50410 70902 70100 70700 20150 Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 84 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips Enterobacteriaceae Non Enteric Gram Negative rods Staphylococcus Streptococcus Neisseria & Haemophilus Corynebacterium Campylobacter Anaerobes Listeria Bacillus Lactobacillus Yeasts Enzymes ID 32 Strips Consumables & Accessories Etest ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer ® QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification API & RapiD Strips (page 7 of 7) Yeasts API 20 C AUX 20210 70700 411004 410589 API 20 C AUX Suspension medium (2ml) LyfoCults® Plus API 20 C AUX Streamlined QC set ‡ LyfoCults Plus API 20 C AUX Comprehensive QC set ‡ 25 Strips & media 100 Ampoules 4 Vials 6 Vials API Candida 10 Strips & media API ZYM API ZYM A API ZYM B 25 Strips 2 x 8ml Ampoules 2 x 8ml Ampoules API Candida 10500 Enzymes API ZYM 25200 70494 70493 ‡ See LyfoCults Plus page 41 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 85 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips ID 32 Strips Enterobacteriaceae Staphylococcus Streptococcus Anaerobes Yeasts Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification ID 32 Strips (page 1 of 2) Enterobacteriaceae ID 32 E 32400 70542 70100 20070 55635 ID 32 E JAMES Reagent Mineral oil NaCl 0.85% medium (2ml) Oxidase Reagent 25 Strips 2 x 5ml Ampoules 125ml Bottle 100 Ampoules 50 Ampoules x 0.75ml rapid ID 32 E (Identification in 4 hours) 32700 70510 70542 70100 20070 55635 410619 rapid ID 32 E API BCP (for automatic reading only) JAMES Reagent Mineral oil NaCl 0.85% medium (2ml) Oxidase Reagent LyfoCults® Plus rapid ID 32 E QC set ‡ 25 Strips 5ml Bottle 2 x 5ml Ampoules 125ml Bottle 100 Ampoules 50 Ampoules x 0.75ml 6 Vials Staphylococcus ID 32 Staph 32500 70562 70442 70572 70100 70700 ID 32 STAPH API FB API NIT 1 & NIT 2 API VP A & VP B Mineral oil Suspension medium (2ml) 25 Strips 2 x 5ml Ampoules 2 x 5ml NIT 1; 2 x 5ml NIT 2 1 x 5ml VP A; 1 x 5ml VP B 125ml Bottle 100 Ampoules ‡ See LyfoCults Plus page 44 for strains contained within the set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 86 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Identification Software API & RapiD Strips ID 32 Strips Enterobacteriaceae Staphylococcus Streptococcus Anaerobes Yeasts Consumables & Accessories Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation API® & ID 32 Identification ID 32 Strips (page 2 of 2) Streptococcus rapid ID 32 STREP (Identification in 4 hours) 32600 70562 70491 70572 70100 70700 rapid ID 32 STREP API FB API NIN API VP A & VP B Mineral oil Suspension medium (2ml) 25 Strips 2 x 5ml Ampoules 2 x 5ml Bottle 1 x 5ml VP A; 1 x 5ml VP B 125ml Bottle 100 Ampoules Anaerobes rapid ID 32 A (Identification in 4 hours) 32300 70562 70442 70542 70100 70700 rapid ID 32 A API FB API NIT 1 & NIT 2 JAMES Reagent Mineral oil Suspension medium (2ml) 25 Strips 2 x 5ml Ampoules 2 x 5ml NIT 1; 2 x 5ml NIT 2 2 x 5ml Ampoules 125ml Bottle 100 Ampoules ID 32 C Suspension medium (2ml) 25 Strips & media 100 Ampoules Yeasts 32200 70700 Consumables & Accessories 70901 70902 99234 15512 99597 70900 70250 70200 70610 70760 Ampoule guards (API/ID 32 range) Ampoule guards (50 CH range) Densimat Densimat Control Kit (for use with Densimat 99234) Lithium batteries (for use with Densimat 99234) McFarland Standard Set PSIpettes (5ml) sterile Stand for 12 ampoules Sterile cotton swabs Sterile loops Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] Pack of 3 Pack of 3 One 5 Ampoules Two 6 Ampoules Pack of 400 12 Ampoule Stand Pack of 100 50 x 10 www.biomerieux.com 87 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description MOLECULAR BIOLOGY 30 Strips 100 Strips 30 Strips Single Push 30 Strips 100 Strips Blister Pack Blister Pack Blister Pack Foil Pack Foil Pack — — — — — — — — — — — 504500 — — — — — — — 506400 — — 532608 — — — — — — — — — 504508 — — — — — — — 506408 — 412219 — 412243 412241 412253 412251 412257 412259 412261 412265 412263 — 412273 412275 412277 412281 412279 412283 412285 — 412289 — — — — — — — — — — — 504510 — — — — — — — — — 501318 — 500918 501018 501518 501818 501618 501718 — 502618 502518 504518 505018 529918 529318 505618 505518 — 506518 — 505818 Etest® Antimicrobial Susceptibility Testing (page 1 of 4) Amikacin (AK 0.016-256) NEW Amoxicillin (ACL 0.002-32 (low)) Amoxicillin (AC 0.016-256 (high)) NEW Amoxicillin-clavulanic acid (2/1) (XL 0.016-256) NEW Ampicillin (AM 0.016-256) NEW Ampicillin-sulbactam (2/1) (AB 0.016-256) AVAILABLE 2013 Azithromycin (AZ 0.016-256) AVAILABLE 2013 Aztreonam (AT 0.016-256) AVAILABLE 2013 Bacitracin (BA 0.016-256) AVAILABLE 2013 Benzylpenicillin (PGL 0.002-32 (low)) Benzylpenicillin (PG 0.016-256 (high)) Cefaclor (CF 0.016-256) Cefepime (PM 0.016-256) AVAILABLE 2013 Cefixime (IX 0.016-256) AVAILABLE 2013 Cefoperazone/sulbactam (2/1) (CPS 0.016-256) AVAILABLE 2013 Cefotaxime (CTL 0.002-32 (low)) NEW Cefotaxime (CT 0.016-256 (high)) NEW Cefotetan (CN 0.016-256) AVAILABLE 2013 Cefoxitin (FX 0.016-256) AVAILABLE 2013 Cefpirome (CR 0.016-256) Cefpodoxime (PX 0.016-256) AVAILABLE 2013 Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). Foam packaging (storage at max. +8 °C and +22 °C). Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 88 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description MOLECULAR BIOLOGY 30 Strips 100 Strips 30 Strips Single Push 30 Strips 100 Strips Blister Pack Blister Pack Blister Pack Foil Pack Foil Pack 537500 — 527300 536000 535300 — — — — — — 508700 — — — — — — 528900 — — 537508 — 527308 536008 535308 — — — — — — 508708 — — 535208 — — — 528908 — — 412291 412293 — — — 412303 412301 412305 412307 412309 412311 — 412315 412317 — 412324 412326 412328 — 412332 412334 — — — — — — — — — — — 508710 — — — — — — — — — — 506718 — — — 507018 506618 506918 503518 507518 508618 508718 509518 — — 535018 535918 509718 — 531618 510518 Etest® Antimicrobial Susceptibility Testing (page 2 of 4) Ceftaroline (CPT 0.002-32) AVAILABLE 2013 Ceftazidime (TZ 0.016-256) NEW Ceftizoxime (CZ 0.002-32) Ceftrobiprole (BPRL 0.002-32 (low)) Ceftrobiprole (BPR 0.016-256 (high)) Ceftriaxone (TXL 0.002-32 (low)) NEW Ceftriaxone (TX 0.016-256 (high)) AVAILABLE 2013 Cefuroxime (XM 0.016-256) AVAILABLE 2013 Cephalothin (CE 0.016-256) AVAILABLE 2013 Chloramphenicol (CL 0.016-256) AVAILABLE 2013 Ciprofloxacin (CI 0.002-32) NEW Clarithromycin (CH 0.016-256) Clindamycin (CM 0.016-256) NEW Colistin (CO 0.016-256) AVAILABLE 2013 Dalbavancin (DLC 0.002-32) Daptomycin (DPC 0.016-256) AVAILABLE 2013 Doripenem (DOR 0.002-32) AVAILABLE 2013 Doxycycline (DC 0.016-256) AVAILABLE 2013 Enrofloxacin (EF 0.002-32) Ertapenem (ETP 0.002-32) AVAILABLE 2013 Erythromycin (EM 0.016-256) NEW Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). Foam packaging (storage at max. +8 °C and +22 °C). Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 89 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description MOLECULAR BIOLOGY 30 Strips 100 Strips 30 Strips Single Push 30 Strips 100 Strips Blister Pack Blister Pack Blister Pack Foil Pack Foil Pack 529100 — 530200 — — — — 527800 — — 513700 — — — — — 516500 517500 — — 519600 529108 — 530208 — — — — 527808 — — 513708 — — — — — 516508 517508 — — 519608 — 412358 — 412364 412368 412366 412374 — 412393 412396 — 412402 412404 412409 412411 412417 — — 412426 412428 — — — 530210 — — — — 527810 — — — — — — — — — 517510 — — 519610 — 511518 530218 530318 512518 — 513618 527818 527418 531318 — 513818 530018 516018 529018 — — 517518 — — 519618 Etest® Antimicrobial Susceptibility Testing (page 3 of 4) Fosfomycin (FM 0.064-1024) Fusidic Acid (FU 0.016-256) AVAILABLE 2013 Gatifloxacin (GA 0.002-32) Gemifloxacin (GEM 0.002-32) AVAILABLE 2013 Gentamicin (GML 0.016-256 (low)) NEW Gentamicin (GM 0.064-1024 (high)) AVAILABLE 2013 Imipenem (IP 0.002-32) Kanamycin (KM 0.016-256) Levofloxacin (LE 0.002-32) NEW Linezolid (LZ 0.016-256) NEW Mecillinam (MM 0.016-256) Meropenem (MP 0.002-32) Metronidazole (MZ 0.016-256) NEW Minocycline (MC 0.016-256) AVAILABLE 2013 Moxifloxacin (MX 0.002-32) AVAILABLE 2013 Mupirocin (MU 0.064-1024) AVAILABLE 2013 Nalidixic Acid (NA 0.016-256) Netilmicin (NC 0.016-256) Nitrofurantoin (NI 0.032-512) AVAILABLE 2013 Norfloxacin (NX 0.016-256) AVAILABLE 2013 Ofloxacin (OF 0.002-32) Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). Foam packaging (storage at max. +8 °C and +22 °C). Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 90 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Page 1 of 4 Page 2 of 4 Page 3 of 4 Page 4 of 4 Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description MOLECULAR BIOLOGY 30 Strips 100 Strips 30 Strips Single Push 30 Strips 100 Strips Blister Pack Blister Pack Blister Pack Foil Pack Foil Pack — — — — 528700 — — — 535100 — — 534500 535600 — — — — 533100 — — — — — — — 528708 — — — 535108 — — 534508 535608 — — — — 533108 — — — 412432 412436 412434 412438 — 412450 412452 412454 — 412458 412461 — — 412471 412473 412475 412479 — 412483 412481 412488 — — — — 528710 — — — — — — — — — — — — — — — — 520518 521518 521418 — 528718 526018 529218 — — 534118 522018 — — 522518 522618 533518 522718 — 523618 524418 525518 Etest® Antimicrobial Susceptibility Testing (page 4 of 4) Oxacillin (OX 0.016-256) NEW Piperacillin (PP 0.016-256) AVAILABLE 2013 Piperacillin-tazobactam (4 mg/L) (PTc 0.016-256) NEW Polymyxin B (PO 0.064-1024) AVAILABLE 2013 Quinupristin-dalfopristin (QDA 0.002-32) Rifampicin (RI 0.002-32) AVAILABLE 2013 Spectinomycin (SC 0.064-1024) AVAILABLE 2013 Streptomycin (SM 0.064-1024) AVAILABLE 2013 Sulbactam (SUL 0.016-256) Sulfamethoxazole (SX 0.064-1024) AVAILABLE 2013 Teicoplanin (TP 0.016-256) NEW Telavancin (TLV 0.002-32) Temocillin (TMO 0.064-1024) * Tetracycline (TC 0.016-256) NEW Ticarcillin-clavulanic acid (2 mg/L) (TLc 0.016-256) AVAILABLE 2013 Tigecycline (TGC 0.016-256) NEW Tobramycin (TML 0.016-256 (low)) AVAILABLE 2013 Tobramycin (TM 0.064-1024 (high)) Trimethoprim (TR 0.002-32) AVAILABLE 2013 Trimethoprim-sulphamethoxazole (1/19) (TS 0.002-32) AVAILABLE 2013 Vancomycin (VA 0.016-256) Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). Foam packaging (storage at max. +8 °C and +22 °C). * For investigational use only, not for clinical use. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 91 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description MOLECULAR BIOLOGY MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY 30 Strips 100 Strips 30 Strips Single Push 30 Strips 100 Strips Blister Pack Blister Pack Blister Pack Foil Pack Foil Pack — 532000 — — — — 525900 535700 — — — 532008 — — — — 525908 535708 — — 412249 — 412269 412350 412352 412380 — — 412440 412490 — — — — — — 525910 — — — 526318 — 532418 510818 510918 525818 525918 — 532118 532818 527700 527500 527900 — — — — — — — — — — — — Etest® Antifungal Susceptibility Testing Amphotericin B (AP 0.002-32) AVAILABLE 2013 Anidulafungin (AND 0.002-32) Caspofungin (CS 0.002-32) AVAILABLE 2013 Fluconazole (FL 0.016-256) AVAILABLE 2013 Flucytosine (FC 0.002-32) AVAILABLE 2013 Itraconazole (IT 0.002-32) AVAILABLE 2013 Ketoconazole (KE 0.002-32) Micafungin (MYC 0.002-32) Posaconazole (POS 0.002-32) AVAILABLE 2013 Voriconazole (VO 0.002-32) AVAILABLE 2013 Antimycobacterial Susceptibility Testing Ethambutol (EB 0.016-256) Ethionamide (ET 0.016-256) Isoniazide (IZ 0.016-256) Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). Foam packaging (storage at max. +8 °C and +22 °C). Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 92 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY 30 Strips Blister Pack 100 Strips Blister Pack 30 Strips Single Push Blister Pack — — 412338 — — 412336 — — 412340 534200 411361 534208 411362 — — 537200 537208 — 537100 537108 — IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer Description Etest® Confirmation of Extended Spectrum Beta-Lactamase (ESBL) ESBL - Cefepime/Cefepime + Clavulanic acid (4 mg/L) AVAILABLE 2013 (PM/PML 0.25-16/0.064-4) ESBL - Cefotaxime/Cefotaxime + Clavulanic acid (4 mg/L) AVAILABLE 2013 (CT/CTL 0.25-16/0.016-1) ESBL - Ceftazidime/Ceftazidime + Clavulanic acid (4 mg/L) AVAILABLE 2013 (TZ/TZL 0.5-32/0.064-4) Detection of Metallo Beta-Lactamase (MBL) MBL - Imipenem/Imipenem + EDTA (IP/IPI 256/1-64) MBL - Meropenem/Meropenem + EDTA (MP/MPI 8/0.032-2) Glycopeptide Resistance Detection (GRD) GRD - Vancomycin/Teicoplanin (VA/TP 0.5-32/0.5-32) AmpC Detection AmpC - Cefotetan/Cefotetan + Cloxacillin (CN/CNI 0.5-32/0.5-32) * Reference Description Presentation Cefinase™ for Detection of Beta-Lactamase 55622 Cefinase Pack of 50 Blister packaging (storage at max. -20 °C). Single Push Blister packaging (storage at max. -20 °C to +8 °C). * For investigational use only, not for clinical use. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 93 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Etest® Accessories 559807 559806 559809 559709 559904 411200 559801 559902 559901 559900 501603 Applicator (1 applicator and 5 strips of tape) Applicator tape Clamps - For Resealing Etest Package Desiccant Capsules Measuring Sticks MiniGrip Vacuum Pen Storage Box, Maxi (12 trays, 12 desiccant strips, 1 applicator, 1 strip of tape and an instruction manual) Storage Box, Maxi (12 desiccant strips, 1 applicator, 1 strip of tape and an instruction manual) Storage Box, Midi (6 desiccant strips, 1 applicator, 1 strip of tape and an instruction manual) Storage Box, Mini (2 desiccant strips, 1 applicator, 1 strip of tape and an instruction manual) Storage Container Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com Pack Pack Pack Pack One of 5 of 5 of 8 of 5 One One One One One One 94 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® Antimicrobial Susceptibility Testing Antifungal Susceptibility Testing Antimycobacterial Susceptibility Testing Confirmation of Extended Spectrum Beta-Lactamase (ESBL) Detection of Metallo Beta-Lactamase (MBL) Glycopeptide Resistance Detection (GRD) AmpC Detection Cefinase™ for Detection of Beta-Lactamase Accessories Biotools™ Biotools Accessories ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Etest® Biotools™ 559802 559804 559803 Simplex C76 - For Automatic Application of Etest Strips Nema C88 - Vacuum Pen For Application of Etest Strips Retro C80 - For Inoculation of Agar Plates One One One Biotools Accessories 5100700700 5100700000 5100700200 5100700300 5100700500 5100599300 5100700400 Biotools AC Adapter Biotools Cord Europe Biotools Petridish Holder 90mm Biotools Petridish Holder 150mm Nema C88 Vacuum Pen Set Nema/Simplex Suction Cups Simplex C76 Tray Holder Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] One One One One One Pack of 2 One www.biomerieux.com 95 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing ATB Galleries Gram Negative rods Pseudomonas & non-Fermenters Urines Staphylococcus Streptococcus Enterococcus Haemophilus Anaerobes Yeasts Veterinary Isolates Consumables & Accessories VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation ATB™ Susceptibility Testing ATB Galleries (page 1 of 2) Gram Negative rods 14315 14960 70700 410997 ATB G- 5 ◊ ATB medium Suspension medium (2ml) LyfoCults® Plus ATB Gram Negative QC set ‡ 25 Strips 100 Ampoules 100 Ampoules 6 Vials Pseudomonas & non-Fermenters 14345 14960 70700 ATB PSE 5 ◊ ATB medium Suspension medium (2ml) 25 Strips 100 Ampoules 100 Ampoules Urines 14335 ATB UR 5 ◊ 25 Strips Staphylococcus 14325 14960 70700 ATB STAPH 5 ◊ ATB medium Suspension medium (2ml) 25 Strips & ATB Na medium 100 Ampoules 100 Ampoules Streptococcus 14355 70700 ATB STREP 5 (Streps & Pneumos ONLY) ◊ Suspension medium (2ml) 10 Strips & ATB S medium 100 Ampoules ATB ENTEROC 5 (Enterococci ONLY) ◊ Suspension medium (2ml) 25 Strips & ATB S medium 100 Ampoules Enterococcus 14365 70700 ‡ See LyfoCults Plus page 45 for strains contained within the set. ◊ For full details of strip compositions please call your local Product Specialist. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 96 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing ATB Galleries Gram Negative rods Pseudomonas & non-Fermenters Urines Staphylococcus Streptococcus Enterococcus Haemophilus Anaerobes Yeasts Veterinary Isolates Consumables & Accessories VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation ATB™ Susceptibility Testing ATB Galleries (page 2 of 2) Haemophilus 14299 20070 410996 ATB HAEMO (Haemophilus + Branhamella ONLY) NaCl 0.85% medium (2ml) LyfoCults® Plus ATB HAEMO QC set ‡ 10 Strips + ATB S medium 100 Ampoules 2 Vials Anaerobes 14269 20070 410999 ATB ANA ◊ NaCl 0.85% medium (2ml) LyfoCults Plus ATB ANA QC set ‡ 10 Strips and ATB S medium 100 Ampoules 2 Vials Yeasts 14204 20070 410998 ATB FUNGUS 3 NaCl 0.85% medium (2ml) LyfoCults Plus ATB FUNGUS QC set ‡ 25 Strips and ATB F2 medium 100 Ampoules 4 Vials Veterinary Isolates 14289 14960 14931 70700 ATB VET (G+ and G- veterinary isolates) ◊ ATB medium ATB S medium Suspension medium (2ml) 25 Strips 100 Ampoules 10 Ampoules 100 Ampoules ‡ See LyfoCults Plus page 45 for strains contained within each set. ◊ For full details of strip compositions please call your local Product Specialist. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 97 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing ATB Galleries Gram Negative rods Pseudomonas & non-Fermenters Urines Staphylococcus Streptococcus Enterococcus Haemophilus Anaerobes Yeasts Veterinary Isolates Consumables & Accessories VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation ATB™ Susceptibility Testing Consumables & Accessories 70901 70902 14960 14931 70700 70640 99234 15512 99597 70900 70250 70200 70610 70760 Ampoule guards (API/ID 32 range) Ampoule guards (50 CH range) ATB medium ATB S medium Suspension medium (2ml) ATB Suspension medium (3ml) Densimat Densimat Control Kit (for use with Densimat 99234) Lithium batteries (for use with Densimat 99234) McFarland Standard Set PSIpettes (5ml) sterile Stand for 12 ampoules Sterile cotton swabs Sterile loops Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] Pack of 3 Pack of 3 100 Ampoules 10 Ampoules 100 Ampoules 100 Ampoules One 5 Ampoules Two 6 Ampoules Pack of 400 12 Ampoule Stand Pack of 100 50 x 10 www.biomerieux.com 98 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Analysers Myla™ Middleware Software VITEK 2 Identification Cards VITEK 2 Antibiotic Susceptibility Cards VITEK 2 Other Consumables & Accessories VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2: Automated Method VITEK 2 Analysers 27226 27228 27202 413641 VITEK 2 Configuration VITEK 2 XL Configuration VITEK 2 additional module OBSERVA® Data Management System Instrument Instrument Instrument One Myla™ Middleware Software 411401 NEW 411613 NEW 410152 NEW Myla Middleware Myla VITEK 2 Connection Kit Myla Multiuser Licence Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] One 1 Kit One Licence www.biomerieux.com 99 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Analysers Myla™ Middleware Software VITEK 2 Identification Cards Gram Negatives (Enterobacteriaceae / non-fermenters) Gram Positive Neisseria, Haemophilus & Fastidious Bacteria Anaerobes Yeasts VITEK 2 Antibiotic Susceptibility Cards VITEK 2 Other Consumables & Accessories VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2: Automated Method VITEK 2 Identification Cards Gram Negatives (Enterobacteriaceae / non-fermenters) 21341 410586 410601 VITEK 2 GN LyfoCults® Plus VITEK 2 GN Streamlined QC set ‡ LyfoCults Plus VITEK 2 GN Comprehensive QC set ‡ 20 Cards 4 Vials 20 Vials VITEK 2 GP LyfoCults Plus VITEK 2 GP Streamlined QC set ‡ LyfoCults Plus VITEK 2 GP Comprehensive QC set ‡ 20 Cards 4 Vials 18 Vials Gram Positive 21342 410616 410584 Neisseria, Haemophilus & Fastidious Bacteria 21346 410628 410611 VITEK 2 NH LyfoCults Plus VITEK 2 NH Streamlined QC set ‡ LyfoCults Plus VITEK 2 NH Comprehensive QC set ‡ 20 Cards 2 Vials 18 Vials VITEK 2 ANC LyfoCults Plus VITEK 2 ANC Streamlined QC set ‡ LyfoCults Plus VITEK 2 ANC Comprehensive QC set ‡ 20 Cards 4 Vials 14 Vials VITEK 2 YST LyfoCults Plus VITEK 2 YST Streamlined QC set ‡ LyfoCults Plus VITEK 2 YST Comprehensive QC set ‡ 20 Cards 2 Vials 22 Vials Anaerobes 21347 410604 410605 Yeasts 21343 410612 410608 ‡ See LyfoCults Plus pages 35 to 39 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 100 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Analysers Myla™ Middleware Software VITEK 2 Identification Cards VITEK 2 Antibiotic Susceptibility Cards Gram Negative rods Gram Positive cocci Yeasts VITEK 2 Other Consumables & Accessories VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2: Automated Method VITEK 2 Antibiotic Susceptibility Cards Supporting EUCAST Gram Negative rods Nordic 412938 413080 413081 413083 413147 410609 VITEK 2 AST-N209 VITEK 2 AST-N217 VITEK 2 AST-N218 VITEK 2 AST-N222 VITEK 2 AST-N230 LyfoCults® Plus VITEK 2 AST-GN QC set ‡ 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 8 Vials VITEK 2 AST-P594 (Replaced by 414576 by end of 2012) VITEK 2 AST-P629 LyfoCults Plus VITEK 2 AST-GP QC set ‡ 20 Cards 20 Cards 16 Vials VITEK 2 AST-P576 VITEK 2 AST-P580 VITEK 2 AST-P586 VITEK 2 AST-P592 VITEK 2 AST-P5XXX VITEK 2 AST-ST01 (Standard Streptococcus Card) LyfoCults Plus VITEK 2 AST-GP QC set ‡ 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 16 Vials VITEK 2 AST-YS06 LyfoCults Plus VITEK 2 AST-YS QC set ‡ 20 Cards 4 Vials Gram Positive cocci Nordic 22294 414576 NEW 410610 Global 22216 22233 22276 22287 XXXXXX 410028 NEW 410610 Yeasts 412610 NEW 410613 ‡ See LyfoCults Plus page 40 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 101 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Analysers Myla™ Middleware Software VITEK 2 Identification Cards VITEK 2 Antibiotic Susceptibility Cards VITEK 2 Other Consumables & Accessories VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2: Automated Method VITEK 2 Other Consumables & Accessories V1204 V1200 70610 69285 21218 21219 27209 27700 21250 21255 93059 96128 Saline 0.45% Saline Dispenser Sterile cotton swabs Test tubes (uncapped) Saline reservoir bags Pipetter / Diluter accessory kit Smart Carrier Station (SCS) Smart Carrier Cassette DensiCHEK™ Plus DensiCHEK Plus - Standard Reference Kit DensiCHEK - Standard Reference VITEK 2 Smart Box 7L Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 3 x 500ml One Pack of 100 2000 12 x 1000ml 330 Pipette Straws & 1 Tube One One cassette with memory chip One 4 Vials 1 Vial One www.biomerieux.com 102 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2 Compact: Automated Method VITEK 2 Compact Analyser 27560 413641 VITEK 2 Compact 60 OBSERVA® Data Management System Instrument One VITEK 2 Compact Analyser VITEK 2 Compact Identification Cards VITEK 2 Compact Antibiotic Susceptibility Cards VITEK 2 Compact Other Consumables & Accessories VITEK MS: MALDI-TOF Mass Spectrometer Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 103 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK 2 Compact Analyser VITEK 2 Compact Identification Cards Gram Negatives (Enterobacteriaceae / non-fermenters) Gram Positive Neisseria, Haemophilus & Fastidious Bacteria Anaerobes Yeasts VITEK 2 Compact Antibiotic Susceptibility Cards VITEK 2 Compact Other Consumables & Accessories VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2 Compact: Automated Method VITEK 2 Compact Identification Cards Gram Negatives (Enterobacteriaceae / non-fermenters) 21341 410586 410601 VITEK 2 GN LyfoCults® Plus VITEK 2 GN Streamlined QC set ‡ LyfoCults Plus VITEK 2 GN Comprehensive QC set ‡ 20 Cards 4 Vials 20 Vials VITEK 2 GP LyfoCults Plus VITEK 2 GP Streamlined QC set ‡ LyfoCults Plus VITEK 2 GP Comprehensive QC set ‡ 20 Cards 4 Vials 18 Vials Gram Positive 21342 410616 410584 Neisseria, Haemophilus & Fastidious Bacteria 21346 410628 410611 VITEK 2 NH LyfoCults Plus VITEK 2 NH Streamlined QC set ‡ LyfoCults Plus VITEK 2 NH Comprehensive QC set ‡ 20 Cards 2 Vials 18 Vials VITEK 2 ANC LyfoCults Plus VITEK 2 ANC Streamlined QC set ‡ LyfoCults Plus VITEK 2 ANC Comprehensive QC set ‡ 20 Cards 4 Vials 14 Vials VITEK 2 YST LyfoCults Plus VITEK 2 YST Streamlined QC set ‡ LyfoCults Plus VITEK 2 YST Comprehensive QC set ‡ 20 Cards 2 Vials 22 Vials Anaerobes 21347 410604 410605 Yeasts 21343 410612 410608 ‡ See LyfoCults Plus pages 35 to 39 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 104 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK 2 Compact Analyser VITEK 2 Compact Identification Cards VITEK 2 Compact Antibiotic Susceptibility Cards Gram Negative rods Gram Positive cocci Yeasts VITEK 2 Compact Other Consumables & Accessories VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2 Compact: Automated Method VITEK 2 Compact Antibiotic Susceptibility Cards Gram Negative rods Nordic 412938 413080 413081 413083 413147 410609 VITEK 2 AST-N209 VITEK 2 AST-N217 VITEK 2 AST-N218 VITEK 2 AST-N222 VITEK 2 AST-N230 LyfoCults® Plus VITEK 2 AST-GN QC set ‡ 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 8 Vials VITEK 2 AST-P594 (Replaced by 414576 by end of 2012) VITEK 2 AST-P629 LyfoCults Plus VITEK 2 AST-GP QC set ‡ 20 Cards 20 Cards 16 Vials VITEK 2 AST-P576 VITEK 2 AST-P580 VITEK 2 AST-P586 VITEK 2 AST-P592 VITEK 2 AST-P5XXX VITEK 2 AST-ST01 (Standard Streptococcus Card) LyfoCults Plus VITEK 2 AST-GP QC set ‡ 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 20 Cards 16 Vials VITEK 2 AST-YS06 LyfoCults Plus VITEK 2 AST-YS QC set ‡ 20 Cards 4 Vials Gram Positive cocci Nordic 22294 414576 NEW 410610 Global 22216 22233 22276 22287 XXXXXX 410028 NEW 410610 Yeasts 412610 NEW 410613 ‡ See LyfoCults Plus page 40 for strains contained within each set. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 105 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK 2 Compact Analyser VITEK 2 Compact Identification Cards VITEK 2 Compact Antibiotic Susceptibility Cards VITEK 2 Compact Other Consumables & Accessories VITEK MS: MALDI-TOF Mass Spectrometer QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® 2 Compact: Automated Method VITEK 2 Compact Other Consumables & Accessories V1204 V1200 70610 69285 V1221 30507 V1222 30501 21250 21255 93059 96128 Saline 0.45% Saline Dispenser Sterile cotton swabs Test tubes (uncapped) Pipette 145 µl Pipette tips (0.5-250 µl) Non-sterile Pipette 280 µl Pipette tips (100-1000 µl) Non-sterile DensiCHEK™ Plus DensiCHEK Plus - Standard Reference Kit DensiCHEK - Standard Reference VITEK 2 Smart Box 7L Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 3 x 500ml One Pack of 100 2000 One 10 Racks x 96 tips One 10 Racks x 60 tips One 4 Vials 1 Vial One www.biomerieux.com 106 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION IDENTIFICATION & SUSCEPTIBILITY TESTING Orientation Tests Rapid Antigen Detection & Identification API® & ID 32 Identification Etest® ATB™ Susceptibility Testing VITEK® 2: Automated Method VITEK 2 Compact: Automated Method VITEK MS: MALDI-TOF Mass Spectrometer VITEK MS Analyser & Accessories VITEK MS Consumables QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation VITEK® MS: MALDI-TOF Mass Spectrometer VITEK MS Analyser & Accessories 410895 411655 411402 VITEK MS Mass Spectrometer with Myla™ VITEK MS Prep Station VITEK MS-ID database Instrument Accessory Accessory VITEK MS Consumables 410893 411071 411072 411721 NEW Target Slides CHCA Matrix Solution Extraction fluid for yeasts VITEK MS Silica Gel Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 32 Disposable Slides (8 x 4) 5 x 0.5ml 5 x 0.5ml 500g www.biomerieux.com 107 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests VIDAS® – Simplicity, Flexibility & Accuracy As a health care provider, you face new challenges every day. VIDAS is constantly adapting to help you overcome them by providing simplicity, flexibility and accuracy. The VIDAS kits include all reagents in a single-dose and ready-to-use format together with separate standards and controls. Making it easy and cost-effective to run one or several patient samples at a time. Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Reference Description Presentation VIDAS VIDAS Instruments 410416 410417 mini VIDAS® Blue System VIDAS Blue System VIDAS Consumables & Accessories 99091 99087 99187 99098 99099 93152 99746 99747 30531 mini VIDAS Printer paper VIDAS decontamination sponges VIDAS MLE card holder VIDAS Loading tray mini VIDAS Loading tray mini VIDAS bar code reader VIDAS Blue bar code reader mini VIDAS Blue bar code reader Dacron swabs Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 5 Rolls per Pack Pack of 150 One One One One One One 100 x 2 www.biomerieux.com 108 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Reference Description Presentation VIDAS® VIDAS Reagents for Clinical Specimens (page 1 of 4) Hepatitis A 30307 30312 VIDAS HAV IgM VIDAS HAV total 30 Tests 30 Tests Hepatitis B 30314 30238 30439 30305 30317 30315 VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS Anti-HBc Total II Anti-HBs Quick HBc IgM II HBe/Anti-HBe HBs Ag Ultra confirmation HBs Ag Ultra 60 Tests 60 Tests 30 Tests 30 Tests 1 x 0.5ml 60 Tests Hepatitis C 30308 VIDAS HCV 60 Tests VIDAS VIDAS VIDAS VIDAS HIV DUO Ultra HIV DUO Quick p24 II p24 Confirmation 60 60 30 60 Tests Tests Tests Tests VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS CMV IgG CMV IgM CMV IgG Avidity RBG II RUB IgM TOXO Competition TOXO IgG II TOXO IgM TOXO Avidity 60 30 30 60 30 60 60 60 30 Tests Tests Tests Tests Tests Tests Tests Tests Tests HIV 30443 30447 30117 30444 TORC 30204 30205 30203 30221 30214 30211 30210 30202 30222 Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 109 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Reference Description Presentation VIDAS® VIDAS Reagents for Clinical Specimens (page 2 of 4) Other Serology 30235 30237 30236 30320 30319 30219 30218 30217 30192 VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS EBNA IgG EBV VCA IgM EBV VCA/EA IgG Lyme IgG Lyme IgM Measles IgG Mumps IgG Varicella Zoster IgG H. pylori IgG 30 30 30 60 60 60 60 60 30 Tests Tests Tests Tests Tests Tests Tests Tests Tests Sepsis 30450 VIDAS B.R.A.H.M.S. Procalcitonin 60 Tests VIDAS CDA/B VIDAS Rotavirus 60 Tests 60 Tests VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS 30 30 60 60 60 60 60 60 Antigen detection 30118 30107 Thyroid 30461 30462 30402 30459 30403 30404 30400 30441 Anti-TPO Anti-Tg FT3 FT4 T3 T4 TSH TSH3 (Ultra sensitive) Tests Tests Tests Tests Tests Tests Tests Tests Anaemia 30411 VIDAS Ferritin Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 60 Tests www.biomerieux.com 110 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Reference Description Presentation VIDAS® VIDAS Reagents for Clinical Specimens (page 3 of 4) Reproduction / Fertility 30431 30407 30405 30406 30409 30410 30418 VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS Estradiol II FSH HCG LH Progesterone Prolactin Testosterone 60 60 60 60 60 60 30 Tests Tests Tests Tests Tests Tests Tests VIDAS VIDAS VIDAS VIDAS VIDAS VIDAS AFP CA 125 II™ CA 15-3™ CA 19-9™ FPSA TPSA 60 30 30 30 60 30 Tests Tests Tests Tests Tests Tests Tumour markers 30413 30426 30429 30427 30440 30428 Drugs 30603 VIDAS Digoxin 60 Tests VIDAS VIDAS VIDAS VIDAS 30 30 60 60 Cardiovascular 30421 30446 30449 30448 CK MB Myoglobin NT-proBNP Troponin I Ultra Tests Tests Tests Tests Quality Control 30706 VIDAS QCV Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 60 Tests www.biomerieux.com 111 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests Reference Description Presentation VIDAS® VIDAS Reagents for Clinical Specimens (page 4 of 4) Immuno-Haemostatis 30455 30115 30436 VIDAS D-Dimer Exclusion II VIDAS Protein C VIDAS von Willebrand Factor (vWFAg) 60 Tests 30 Tests 30 Tests Serum free (for urinary assay of VIDAS Cortisol) VIDAS Beta-2 Microglobulin VIDAS Cortisol S 1 x 10ml 30 Tests 60 Tests Other tests 66581 30420 30451 Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 112 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY IMMUNOASSAYS VIDAS® VIDAS Instruments VIDAS Consumables & Accessories VIDAS Reagents for Clinical Specimens Hepatitis A Hepatitis B Hepatitis C HIV TORC Other Serology Sepsis Antigen detection Thyroid Anaemia Reproduction / Fertility Tumour markers Drugs Cardiovascular Quality Control Immuno-Haemostatis Other tests Microelisa Microbiology Reagent Kits Hepatitis HIV Syphilis Reference Description Presentation Microelisa Microbiology Reagent Kits Hepatitis 284132 284133 280253 284144 HEPANOSTIKA™ – HBsAg Ultra HEPANOSTIKA – HBsAg Ultra HEPANOSTIKA – HBsAg Ultra Confirmation HEPANOSTIKA – anti-HBc Uni-Form 24 x 8 72 x 8 25 24 x 8 Tests Tests Tests Tests VIRONOSTIKA™ HIV Uni-Form II plus 0 VIRONOSTIKA HIV Uni-Form II plus 0 VIRONOSTIKA HIV Ag/Ab VIRONOSTIKA HIV Ag/Ab 24 72 24 72 Tests Tests Tests Tests TREPANOSTIKA™-TP recombinant TREPANOSTIKA-TP recombinant (includes 1ml positive control, 2ml negative control) 72 x 8 Tests 24 x 8 Tests HIV 284017 284018 259851 259852 x x x x 8 8 8 8 Syphilis 285035 285034 Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 113 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Serodiagnosis Leishmaniasis 75931 Leishmania-Spot IF 10 x 10 Circles Falciparum-Spot IF 10 x 10 Circles Malaria 72751 Rheumatic Fever (Streptococcal Infection) Antistreptodornase B 72331 ASD-Kit 20 Tests ASL-Kit 20 Tests Antistreptolysin 72351 Rheumatoid Factors 72013 Arthri-Slidex 100 Tests Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 114 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Serodiagnosis Syphilis RPR inhibition 280447 RPR - nosticon II 100 Tests Immunofluorescence 75661 75681 Sorbent Trepo-Spot IF 5ml 10 x 10 Circles Positive control serum 72191 Serotrol 1ml Toxoplasmosis Agglutination 75361 79322 75481 Toxo ISAGA Toxo-ISAGA IgA Toxo-Screen DA Kit for 192 Tests 192 Wells : 48 Screening Tests Complete Kit with 4 Microtitration plates Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus Immunofluorescence 75471 Toxo-spot IF™ Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 10 x 10 Circles www.biomerieux.com 115 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Immunology Liquid Globulins for Immunofluorescence 75692 75603 75672 75521 Fluoline-G - anti-human IgG Fluoline-H - anti-human Immunoglobulins Fluoline-M - anti-human IgM Fluoprep - mounting medium 1ml 1ml 1ml 15ml Bottle Bottle Bottle Bottle Labware 75751 Slides of 10 x 6 mm Circles 72 Slides Argene™ Immunology Reagents (page 1 of 4) Herpes Virus Antibodies 14-002 11-002 11-004 11-003 17-090 12-090 11-088 11-089 11-090 17-017 11-018 11-017 11-002G 11-002I Anti CMV CINApool + F(ab')2 FITC Anti CMV CINApool purified Anti HCMV I.E.A + E.A purified Anti HCMV I.E.A purified anti HSV-1 + 2 FITC Anti HSV-1 + 2 FITC concentrated Anti HSV-1 purified Anti HSV-2 purified Anti HSV-1 + 2 purified anti VARICELLA ZOSTER VIRUS FITC Anti VZV 1U1 purified Anti VZV 2013 purified Dilution buffer for CINApool Evans blue 1% for CINApool Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 0.5ml (20x) 0.5ml (20x) 0.5ml (50x) 0.5ml (50x) 80 Tests 7.5ml 7.5ml 7.5ml 7.5ml 80 Tests 0.5ml (40x) 0.5ml (40x) 4.5ml 250µl www.biomerieux.com 116 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Immunology Argene™ Immunology Reagents (page 2 of 4) Respiratory Virus Antibodies 11-091 17-020 12-020 11-020 17-091 17-092 11-030 11-035 17-270 17-030 12-030 17-035 12-035 17-036 11-036 17-037 11-037 17-038 12-038 11-038 17-040 12-040 11-040 17-042 12-042 11-042 Anti ADENO-INFLU-PARA purified anti ADENOVIRUS GROUP FITC Anti ADENOVIRUS GROUP FITC concentrated Anti ADENOVIRUS GROUP purified anti ADENOVIRUS, INFLUENZA, PARAINFLUENZA FITC anti ADENOVIRUS, INFLUENZA, PARAINFLUENZA, RSV FITC Anti FLU A GROUP purified Anti FLU B GROUP purified anti human METAPNEUMOVIRUS FITC anti INFLUENZA A GROUP FITC Anti INFLUENZA A GROUP FITC concentrated anti INFLUENZA B GROUP FITC Anti INFLUENZA B GROUP FITC concentrated anti PARAINFLUENZA 1 FITC Anti PARAINFLUENZA 1 purified anti PARAINFLUENZA 2 FITC Anti PARAINFLUENZA 2 purified anti PARAINFLUENZA 3 FITC Anti PARAINFLUENZA 3 FITC concentrated Anti PARAINFLUENZA 3 purified anti PARAINFLUENZA GROUP FITC Anti PARAINFLUENZA GROUP FITC concentrated Anti PARAINFLUENZA purified anti RSV GROUP FITC Anti RSV GROUP FITC concentrated Anti RSV GROUP purified Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 0.5ml (40x) 80 Tests 0.5ml (40x) 0.5ml (40x) 80 Tests 80 Tests 0.5ml (40x) 0.5ml (40x) 80 Tests 80 Tests 0.5ml (40x) 80 Tests 0.5ml (40x) 80 Tests 0.5ml (40x) 80 Tests 0.5ml (40x) 80 Tests 0.5ml (40x) 0.5ml (40x) 80 Tests 1ml (20x) 0.5ml (40x) 80 Tests 0.5ml (40x) 0.5ml (40x) www.biomerieux.com 117 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Immunology Argene™ Immunology Reagents (page 3 of 4) CINAkit 19-002 19-0028 19-0028US 19-002K 19-002C 33-041 19-002E 19-002B CINAkit complete kit HCMV ppUL83 (pp65) I.F. Antigenemia CINAkit complete kit HCMV ppUL83 (pp65) I.F. Antigenemia CINAkit complete kit HCMV ppUL83 (pp65) I.F. Antigenemia Dextran 6% for CINAkit 19-002 Fixative solution for CINAkit 19-002 Mounting medium for immunofluorescence Permeabilization solution for CINAkit 19-002 Rapid lysis solution for CINAkit 19-002 100 Tests 200 Tests 200 Tests 60ml 110ml 15ml 110ml 45ml Control Slides 40-061 40-022 40-017 40-071 40-081 40-161 40-091 40-101-1 40-111 40-121 40-041 Control Control Control Control Control Control Control Control Control Control Control slide slide slide slide slide slide slide slide slide slide slide ADENOVIRUS CYTOMEGALOVIRUS IEA-EA HERPES SIMPLEX TYPE 1 & 2 INFLUENZA A INFLUENZA B PANEL ADV, IA, IB, VRS, P1, P2, P3 PARAINFLUENZA 1 PARAINFLUENZA 2 PARAINFLUENZA 3 RSV VARICELLA - ZOSTER Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 5 5 5 5 5 5 5 5 5 5 5 www.biomerieux.com Slides Slides Slides Slides Slides Slides Slides Slides Slides Slides Slides 118 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Immunology Argene™ Immunology Reagents (page 4 of 4) Other 14-002 11-033 11-034 12-090 11-045 50-010 50-011 50-012 50-021 50-022 11-046 33-030 51-010 51-011 31-010 31-030 33-041 33-011 Anti CMV CINApool + F(ab')2 FITC Anti HAV 1009 purified Anti HAV 813 purified Anti HSV-1 + 2 FITC concentrated Anti MEASLES purified anti MOUSE IgG + IgM "human ads." FITC anti MOUSE IgG + IgM "human ads." FITC anti MOUSE IgG + IgM "human ads." FITC anti MOUSE IgG + IgM "human ads." PEROXIDASE anti MOUSE IgG + IgM "human ads." PEROXIDASE Anti MUMPS purified Evans blue 1% F(ab')2 anti MOUSE IgG + IgM "human ads." FITC F(ab')2 anti MOUSE IgG + IgM "human ads." FITC IF slides - Teflon coated - 6mm wells "Kova glasstic slides" for antigenemia Mounting medium for immunofluorescence PBS pH 7.2 without Ca2+ and Mg2+ Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 250ug 0.5ml 250ug 0.5ml 0.5ml (40x) 0.5ml 1ml 2ml 1ml 2ml 0.5ml (40x) 2ml 0.5ml 1ml 100 Slides 100 Slides 15ml 4 Litres www.biomerieux.com 119 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence QC ORGANISMS Reference Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Rapid Tests Faecal Occult Blood 410594 414275 414276 Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other CULTURE MEDIA bioNexia® FOBplus Test Rapid (5 minute) immunological point of care test for detection of human haemoglobin in stools bioNexia FOBplus Lab Kit Rapid (5 minute) immunological point of care test for detection of human haemoglobin in stools, kit specially developed for laboratory personnel bioNexia FOBplus Patient Set Patient collection set complementary kit to ref 410594 bioNexia FOBplus Test and 414275 bioNexia FOBplus Lab Kit 25 Tests 25 Tests 25 Tests Bladder Tumour Antigen 410591 bioNexia BTA Test Rapid (5 minute) point of care test for bladder tumour antigen in urine 10 Tests bioNexia Troponin I Test Rapid (10 minute) point of care test for Troponin I in whole blood or serum/plasma 10 Tests bioNexia CRPplus Test Rapid (10 minute), semi-quantitative point of care test for C-Reactive Protein in whole blood 10 Tests bioNexia Influenza A+B Test Rapid (10 minute), test for detection of Influenza A and B in nasopharyngeal aspirates 10 Tests Troponin 410598 C-Reactive Protein 410592 Influenza 410918 All bioNexia kits contain everything needed for sample collection and test processing, e.g. lancets, swabs etc. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 120 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Serodiagnosis Leishmaniasis Malaria Rheumatic Fever (Streptococcal Infection) Antistreptodornase B Antistreptolysin Rheumatoid Factors Syphilis RPR inhibition Immunofluorescence Positive control serum Toxoplasmosis Agglutination Immunofluorescence QC ORGANISMS Reference CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY Description MOLECULAR BIOLOGY Presentation Rapid Tests HIV 31112 VIKIA® HIV 1/2 25 Test Devices VIKIA HBsAg 25 Test Devices Hepatitis B 31124 Rotavirus / Adenovirus 31111 VIKIA Rota/Adeno 20 Test Devices Immunology Liquid Globulins for Immunofluorescence Labware Argene™ Immunology Reagents Herpes Virus Antibodies Respiratory Virus Antibodies CINAkit Control Slides Other Rapid Tests Faecal Occult Blood Bladder Tumour Antigen Troponin C-Reactive Protein Influenza HIV Hepatitis B Rotavirus / Adenovirus Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 121 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables NucliSENS® easyMAG® Automated Extraction System High Extraction Quality Magnetic extraction based on the gold standard BOOM technology (silica particles) High concentration factor: up to 100 High quality RNA and DNA extraction: the easyMAG has been validated with a wide range of clinical commercial kits for real-time amplification and detection Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Flexibility/Versatility Optimise your Workflow and Productivity, having the possibility to perform several applications in the same run: 40 minutes for 24 extractions Generic protocol approach for DNA and RNA extraction One set of reagents whatever the application High flexibility in term of sample input and output volumes Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 122 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation NucliSENS® NucliSENS Magnetic Extraction Systems easyMAG® Automated Extraction System 280140 278303 280141 280151 280146 280130 280131 280132 280133 280134 280135 easyMAG Automated Magnetic Extraction System Strip Plate (Greiner) BioHit Pipettor BioHit adaptor BioHit Pipette tips NucliSENS easyMAG Extraction Buffer 1 NucliSENS easyMAG Extraction Buffer 2 NucliSENS easyMAG Extraction Buffer 3 NucliSENS easyMAG Magnetic Silica NucliSENS easyMAG Lysis Buffer NucliSENS easyMAG Disposables One 100 Plates One One 960 4 x 1 Litre 4 x 1 Litre 4 x 1 Litre 384 Extractions 4 x 1 Litre 384 Positions miniMAG® Semi Automated Extraction System 200305 200299 200292 200293 200294 NucliSENS miniMAG Extractor Magnetic Rack 12 hole NucliSENS Lysis Buffer 2ml NucliSENS Magnetic Extraction Reagents Micro Tubes 1.5ml with cap Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] One One 48 Tubes 48 Tests 500 www.biomerieux.com 123 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab ® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation NucliSENS® NucliSENS EasyQ® System Real Time Amplification & Detection System 285052 285056 270677 EasyQ System for Real Time Amplification and Detection using Molecular Beacons. Includes EasyQ Reader, PC Workstation, Director Software, Incubator, Printer & Tube-strip Centrifuge Tube-strip Centrifuge Mo Bio Adaptor FOR Genie Vortex One One One NucliSENS EasyQ® Reagents, Consumables & Accessories Enterovirus 410311 410312 NucliSENS EasyQ Enterovirus v1.1 NucliSENS EasyQ Enterovirus Controls 48 Tests 6 x Positive and 6 x Negative HSV 280110 280104 NucliSENS EasyQ HSV 1 + 2 NucliSENS EasyQ Basic Kit v2 48 Tests 48 Tests MRSA 280115 280101 NucliSENS EasyQ MRSA Kit COPAN Sterile dry flocked swabs 48 Tests Pack of 100 Consumables 285048 285051 0.2ml 8-tube strips 0.2ml 8-tube caps Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 125 125 www.biomerieux.com 124 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation Argene™ Real-Time PCR (page 1 of 3) Complete Kits 69-010B 69-013B 69-011B 69-100B 69-003B 69-002B 69-005B 69-004B ADENOVIRUS R-gene™ - Quantification kit BK Virus R-gene Quantification Kit BORDETELLA R-gene - Real-Time PCR Kit CMV, HHV6, 7, 8 R-gene Quantification Kit CMV R-gene Quantification Kit EBV R-gene Quantification Kit ENTEROVIRUS R-gene - Real-Time PCR Kit HSV1 HSV2 VZV R-gene Quantification Kit 90 90 60 140 90 90 90 180 Tests Tests Tests Tests Tests Tests Tests Tests 60 60 60 60 60 60 Tests Tests Tests Tests Tests Tests Respiratory Multi Well System 71-043 71-044 71-045 71-040 71-042 71-041 Adenovirus/human Bocavirus r-gene™ Chla/Myco Pneumo r-gene Coronavirus/Parainfluenza virus r-gene Influenza A/B r-gene Rhinovirus, Enterovirus/Cell control r-gene RSV/hMPV r-gene Real-Time PCR Assays for Transplant & Respiratory Medicine Validated for Included All major Real-Time All controls to monitor the PCR Instruments entire process Multiple automatic Ready-to-use reagents extraction systems Quantification standards Various types of samples Turnkey solution Uniform procedure Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 125 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation Argene™ Real-Time PCR (page 2 of 3) Optimised Primers & Probes 71-010 71-005 71-009 71-012 71-001 69-100R6 69-100R7 69-100R8 71-008 71-015 71-016 71-300 71-004 71-003 71-002 71-007 71-017 Adenovirus r-gene™ Primers/Probes BK Virus r-gene Primers/Probe Bocavirus r-gene Primers/Probe Bordetella parapertussis r-gene Chlamydophila pneumoniae r-gene Primers/Probe HHV6 amplification premix HHV7 amplification premix HHV8 amplification premix hMPV A/B r-gene Primers/Probes HSV1 r-gene HSV2 r-gene Influenza A(M) Group & H1N1 2009 r-gene JC Virus r-gene Primers/Probe Legionella species r-gene Primers/Probe Mycoplasma pneumoniae r-gene Primers/Probe RSV A/B r-gene Primers/Probes VZV R-gene™ Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 60 60 60 60 60 60 20 20 60 60 60 60 60 60 60 60 www.biomerieux.com Reactions Reactions Reactions Reactions Reactions Reactions Reactions Reactions Reactions Reactions Reactions 60 Tests Reactions Reactions Reactions Reactions Reactions 126 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation Argene™ Real-Time PCR (page 3 of 3) Hybridowell™ 67-065BC 67-025BC 67-080BC 67-090BC 67-050 67-012 67-130BC ADENOVIRUS CONSENSUS amplification/detection CHLAMYLEGE amplification/detection ENTEROVIRUS CONSENSUS amplification/detection HERPES CONSENSUS GENERIC amplification/detection Hybridowell HERPES Identification (for 67-090BC) Hybridowell Universal, complete kit JC/BK CONSENSUS amplification/detection 50 50 50 50 Samples Samples Samples Samples 96 Tests 96 Tests 50 Samples Ancillary Reagents 68-009 71-106 68-020 71-103 71-100 71-101 68-006 68-008 71-105 65-001 Bocavirus r-gene™ positive control CELL Control r-gene CMV Clear QC Panel Colour Compensation r-gene DICO Ampli r-gene DICO Extra r-gene Quanti FluA QS r-gene Quanti HHV8 QS r-gene RICO Extra r-gene UNG r-gene Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 10 Reactions 100 Reactions 3 x 1ml 3 x 60µl 100 Reactions 100 Reactions 30 Reactions 30 Reactions 100 Reactions 500 Tests www.biomerieux.com 127 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories DiversiLab® – Automated Strain Typing DiversiLab is an automated platform using rep-PCR technology that provides standardised, reproducible DNA fingerprinting of bacterial and fungal samples for complete isolate characterisation. It rapidly and accurately distinguishes your isolates to strain level. With DiversiLab technology, strain typing becomes much easier and possible in routine use, even with numerous specimens. Intra-laboratory and Inter-laboratory reproducibility are key to accurately track HAI. The DiversiLab platform has been shown to have: <1% variability for inter-laboratory reproducibility <2% variability for intra-laboratory reproducibility The synergy between this innovative genotyping system and bioMérieux’s other systems supports clinical decisions that can ultimately reduce the number of HAI and ensure the appropriate use of antibiotic therapy. Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 128 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation DiversiLab® DiversiLab Equipment DiversiLab System Instrument DiversiLab Fingerprinting Kits 410946 410948 410949 410962 411007 410963 410964 410965 410966 410967 410968 410969 410980 410993 411008 410981 410982 410983 410984 410986 410985 410987 410988 410994 410989 410990 410991 410992 DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab DiversiLab Acinetobacter Kit Archaeabacteria Kit Aspergillus Kit Bacillus Kit Bacteria (Gram Positive & Gram Negative) Kit Bifidobacterium (& Rhodococcus & Nocardi spp) Kit Campylobacter Kit Candida Kit Clostridium Kit Clostridium perfringens Kit Enterobacter Kit Enterococcus Kit Escherichia (& Shigella spp) Kit Francisella Kit Fungal (Yeasts & Molds) Kit Klebsiella Kit Lactobacillus Kit Listeria Kit Mycobacterium Kit Mycobacterium tuberculosis Kit Mycoplasma Kit Propionibacterium (& Legionella spp) Kit Pseudomonas Kit Saccharomyces Kit Salmonella Kit Serratia Kit Staphylococcus Kit Streptococcus Kit Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 24 www.biomerieux.com Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests Tests 129 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY MOLECULAR BIOLOGY MOLECULAR BIOLOGY NucliSENS® Feature – NucliSENS easyMAG® NucliSENS Magnetic Extraction Systems easyMAG Automated Extraction System miniMAG® Semi Automated Extraction System NucliSENS EasyQ® System Real Time Amplification & Detection System NucliSENS EasyQ Reagents, Consumables & Accessories Enterovirus HSV MRSA Consumables Argene™ Real-Time PCR Complete Kits Repiratory Multi Well System Optimised Primers & Probes Hybridowell™ Ancillary Reagents DiversiLab® Feature – DiversiLab DiversiLab Equipment DiversiLab Fingerprinting Kits DiversiLab Test Accessories DiversiLab Extraction Accessories Reference Description Presentation DiversiLab® DiversiLab Test Accessories 270670 270715 270673 270656 411164 DiversiLab LabChip Kit 25 Chips Kit contains: DiversiLab LabChip Reagents DiversiLab LabChip DiversiLab Syringe DiversiLab LabChip Priming Station DiversiLab Gel Standards DiversiLab Thermal Cycler QC Kit DiversiLab Training Kit 325 Tests 325 Tests 25 LabChips x 13 Tests Two One 1 Set 48 Tests 24 Tests DiversiLab Extraction Accessories 270676 270675 270677 DiversiLab Mo Bio UltraClean™ DiversiLab Mo Bio UltraClean Mo Bio Adaptor FOR Genie Vortex 250 Tests 50 Tests One DiversiLab v3.0 PC #3 Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] www.biomerieux.com 130 HOME HOW BLOOD CULTURE / FULL MICROBIOLOGY TO USE MYCOBACTERIAL CULTURE LAB AUTOMATION QC ORGANISMS CULTURE MEDIA IDENTIFICATION & SUSCEPTIBILITY TESTING IMMUNOASSAYS Denmark: [email protected] Finland: [email protected] Norway: [email protected] Sweden: [email protected] MANUAL SEROLOGY / RAPID TEST / IMMUNOLOGY www.biomerieux.com MOLECULAR BIOLOGY 131