Download - VAC Ulta
Transcript
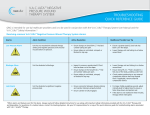
DISCLAIMER: This is intended for use as a supplement. Please refer to the Instructions for Use and User Manual for full safety information. V.A.C.Ulta™ Negative Pressure Wound Therapy Unit with V.A.C.® Therapy V.A.C.Ulta™ Therapy Unit Setting up the Therapy Unit 1. Attach the canister Power Button Canister Release Button Canister 22 Touch Screen V.A.C.® Therapy: 500 mL or 1000 mL canister (Acute Care only) 2. Connect the Dressing Tubing (after applying the dressing) Align, Push, and Twist Data Door (Connection Ports) Battery Charging Indicator Light NOTE: Refer to the Instructions for Use for complete details regarding Dressing Application. Initiating V.A.C.® Therapy Choose Therapy screen V.A.C.® Therapy Settings screen Confirm Settings screen SealCheck™ Leak Detector screen Press and hold power button until light comes on. V.A.C.® Therapy Begins Select V.A.C.® Therapy Configure V.A.C.® Therapy as prescribed. Choose Continuous (default) setting and select OK. See below for DPC* option Select OK to accept settings Enhanced Features Seal Check™ Leak Detector V.A.C.® Therapy Home screen Contraindications Dynamic Pressure Control™ * V.A.C.® Therapy Settings screen Do not place foam dressings of the V.A.C.Ulta™ Therapy System (including both V.A.C.® Therapy and V.A.C. VeraFlo™ Therapy Dressings) directly in contact with exposed blood vessels, anastomotic sites, organs, or nerves. V.A.C.® Therapy is contraindicated for patients with: • Malignancy in the wound • Untreated osteomyelitis • Non-enteric and unexplored fistulas • Necrotic tissue with eschar present (NOTE: After debridement of necrotic tissue and complete removal of eschar, V.A.C.® Therapy may be used.) • Sensitivity to silver (V.A.C. GranuFoam Silver® Dressing only) NOTE: Refer to the Warnings and Precautions section of the Safety Information Sheet for additional information including details on potential risks associated with bleeding and infection. For complete information on indications, contraindications, and other safety information please reference the User Manual and Safety Information Sheet. Configure V.A.C® Therapy as prescribed and select OK. For more information, please contact your KCI Representative: _____________________________ at _________________________ NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a physician and product instructions for use prior to application. Rx only. Important: This quick reference is provided as a basic overview only. Refer to the V.A.C.Ulta™ User Manual and Safety Information Sheet for detailed instructions, including important safety information. For medical emergencies, consult treating physician or call emergency services. Carefully read and follow the KCI V.A.C.® Therapy Clinical Guidelines and all literature and labeling accompanying product. Contact KCI at 1-800-275-4524 if you have any questions before initiating therapy. Additional information may be available at www.VACUlta.com. Rx only. ©2012 KCI Licensing, Inc. All rights reserved. Third party trademarks are the property of their respective owners. All other trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates and/ or licensors. DSL#14-0456.US • Lit. 29-H-220 • (Rev. 9/14) DISCLAIMER: This is intended for use as a supplement. Please refer to the Instructions for Use and User Manual for full safety information. V.A.C.Ulta™ Negative Pressure Wound Therapy Unit with V.A.C. VeraFlo™ Therapy Setting up the Therapy Unit V.A.C.Ulta™ Therapy Unit 11 Power Button 1. Attach the V.A.C. VeraLink™ Cassette V.A.C. V.A.C. VeraLink™ VeraLink™ Cassette Cassette PivotPivot Connection Connection 22 Solution Container Arm 22 1 Solution Bag 33 V.A.C. VeraLink™ Cassette Pivot Connection 2. Extend the Solution Arm 2 2 3 3 3 Touch Screen Instillation Cassette (V.A.C. VeraLink™ Cassette) 2 2 Canister Release Button 2 2 4 4 Canister 2 Battery Charging Indicator Light Data Door (Connection Ports) 3. Attach the 500 mL or 1000 mL Canister 3 4. Spike and Hang the Solution Container Bag/Bottle Contraindications Do not place foam dressings of the V.A.C.Ulta™ Therapy System (including both V.A.C.® Therapy and V.A.C. VeraFlo™ Therapy Dressings) directly in contact with exposed blood vessels, anastomotic sites, organs, or nerves. 3 4 4 2 2 2 2 3 5. Connect the Dressing Tubing (after applying the dressing) Align, Push, and Twist 3 3 V.A.C.® Therapy and V.A.C. VeraFlo™ Therapy are contraindicated for patients with: • Malignancy in the wound • Untreated osteomyelitis • Non-enteric and unexplored fistulas • Necrotic tissue with eschar present (NOTE: After debridement of necrotic tissue and complete removal of eschar, V.A.C.® Therapy may be used.) • Sensitivity to silver (V.A.C. GranuFoam Silver® Dressing only) NOTE: Refer to the Warnings and Precautions section of the Safety Information Sheet for additional information including details on potential risks associated with bleeding and infection. For complete information on indications, contraindications, and other safety information please reference the User Manual and Safety Information Sheet. Additional Contraindications Specific to V.A.C. VeraFlo™ Therapy: • Do not use V.A.C.® Dressings with Octenisept®*, hydrogen peroxide or solutions that are alcohol-based or contain alcohol. • Do not deliver fluids to the thoracic or abdominal cavity due to the potential risk to alter core body temperature and the potential for fluid retention within the cavity. • Do not use V.A.C. VeraFlo™ Therapy unless the wound has been thoroughly explored due to the potential for inadvertent instillation of topical wound solutions to adjacent body cavities. Connect the Instillation Line and then the V.A.C.® Therapy Line NOTE: Refer to the Instructions for Use for complete details regarding Dressing Application. Initiating V.A.C. VeraFlo™ Therapy Not available in the United States. Brand name referenced is not a trademark of KCI, its affiliates, or licensors. * V.A.C. VeraFlo Settings screen ™ Choose Therapy screen Confirm Settings screen Press and hold power button until light comes on. V.A.C. VeraFlo™ Therapy Begins Select V.A.C. VeraFlo™ Therapy Confirm V.A.C.VeraFlo™ Therapy as prescribed and select OK. Select OK to accept settings Select V.A.C. VeraFlo™ Therapy Configure V.A.C. VeraFlo™ Therapy as prescribed and select OK. Select OK to accept settings Enhanced Features Fill Assist (with test cycle) Seal Check™ Leak Detector Dressing Soak For more information, please contact your KCI Representative: _____________________________ at _________________________ NOTE: Specific indications, contraindications, warnings, precautions and safety information exist for KCI products and therapies. Please consult a physician and product instructions for use prior to application. Rx only. Important: This quick reference is provided as a basic overview only. Refer to the V.A.C.Ulta™ User Manual and Safety Information Sheet for detailed instructions, including important safety information. For medical emergencies, consult treating physician or call emergency services. Carefully read and follow the KCI V.A.C.® Therapy Clinical Guidelines and all literature and labeling accompanying product. Contact KCI at 1-800-275-4524 if you have any questions before initiating therapy. Additional information may be available at www.VACUlta.com. Rx only. ©2012 KCI Licensing, Inc. All rights reserved. Third party trademarks are the property of their respective owners. All other trademarks designated herein are proprietary to KCI Licensing, Inc., its affiliates and/ or licensors. DSL#14-0456.US • Lit. 29-H-220 • (Rev. 9/14)