Download - University of Illinois College of Medicine at Chicago
Transcript
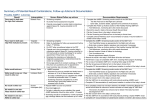
THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 1 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE GUIDELINES FOR UNIVERSAL NEWBORN HEARING SCREENING PROGRAM PURPOSE: To provide guidelines for the early detection of and intervention for infants with congenital hearing loss through a universal newborn hearing screening program (UNHSP). Five essential elements to an effective UNHSP include: initial screening, tracking and follow-up, identification, intervention and evaluation. BACKGROUND/SUMMARY Approximately 5000 infants are born in the US with moderate-to-profound, bilateral permanent hearing loss (PHL), making hearing impairment the most common birth defect affecting newborns. Significant hearing loss is associated with delayed language development and may impact cognition, socialemotional development, and long term educational and employment levels. Prior to 1994, the Joint Committee on infant hearing (JCIH) recommended selective screening of high-risk newborns, which failed to discover up to 50% of the infants with hearing impairments. In addition, the diagnosis was frequently delayed until greater than one to two years of age. Early auditory stimulation during the first few years of life is critical to the development of speech and language skills. In recognition of this critical period of brain development, the JCIH in 1994 endorsed the goal of universal detection of hearing loss of infants by three months of age. Their premise was that earlier application of current therapies (speech and language therapy and amplification devices) might reduce or eliminate the gap in skills between hearing and deaf children. The Joint Committee position was developed and approved by the American Academics of Pediatrics Otolaryngology-Head and Neck Surgery, and Audiology, State Health and Welfare Administrators, and the American Speech-LanguageHearing Association. The 1994 recommendation follows the 1993 National Institute of Health recommendation, in their consensus statement on the early identification of hearing impairment in infants and young children, that every newborn be screened, preferably prior to hospital discharge. METHOD OF NEWBORN HEARING SCREENING Auditory brainstem response (ABR) is an accepted diagnostic standard for evaluating newborn's hearing status, screening for hearing loss of 30dB NHL (decibel normal hearing level) or greater. ABR testing measures the electroencephalographic waves generated from the auditory brainstem. ALGO 5 newborn hearing screener delivers thousands of soft clicks sounds (35 dB nHL) to a newborn's ear through disposable earphones. Each click generates a series of identifiable brain waves from a special area of the baby's brain called the auditory brainstem. This technique is noninvasive, rapid, easy to perform and requires the infant be in a quiet state, but is not affected by middle or external ear debris. The ALGO 5 screener uses advance signal processing techniques to separate the ABR waves from the background noise and other brain activity. The brain waves are average and matched against a pattern, called a template. The screener must detect the ABR waveform and with great statistical accuracy to determine that a response is present. The device automatically produces either a PASS or REFER result that is not subject to user interpretation. A PASS result indicates a 99.96% probability that the newborn has normal hearing. A REFER result indicates that the PASS criteria is not met and further evaluation is needed. The ALGO screener design offers simple user interaction with on-screen instruction. The ALGO is part of a portable workstation and can be used by volunteers and other non-medical personnel (as appropriate). THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 2 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE DEFINITION OF TERMS: • ALGO 5 is a newborn hearing screener that is easy-to -use, portable, noninvasive instrument used to screen infants for hearing loss. • Auditory Brainstem Response (ABR) is a series of identifiable brain waves from a special area of the baby's brain. • Acoustic Transducer Assembly 3 (ATA 3) consists of two cables with colored-coded plastic housing at their end. The housing hold speakers (which generate the click sounds) and microphones (which listen for ambient noise). The red-tipped traducer inserts into the ear couplers disposable earphone for the right ear, the blue-tipped traducer in the left ear. • Patient Cable Assembly 3 (PCA 3) consists of three individual cables, each with a color-coded spring clip at one end that merge into one cable before connecting to the pre-amplifier box and then the instrument. • PCA 3 spring clip attaches to the sensor on the baby's skin and transmits the brain wave signals back to the instrument. • Pre-amplifier box amplifies the sound before reaching the baby's ear. • Flexi Couplers are disposable earphones. The coupler ensures that a uniform level of sound reaches the ear, and isolates the ear from the noise in the environment. • Likelihood Ratio (LR) represents the probability that the infant's response is present when compared to a no-response condition. When the LR reaches 160 or greater, the ALGO 5 screener stops collecting data and displays a pass for the ear being tested. A likelihood ratio of 160 or greater indicates that the infant's response matches the template pattern to a 99.98% or better confidence level. • Sweep is a one instance in which the ALGO 5 screener generates a click and then collects data in response to that click. Only sweeps that are accepted by the ALGO 5 (that is not contaminated by ambient noise, electrical interference, or excessive muscular activity) are counted and accumulated on the display. The sweep count is updated every 10 sweeps. • Sensors are electrodes that allow the minute electrical signals of the auditory brainstem response to be transmitted across the infant's skin to the ALGO 5 screener. • Myogenic is a muscle or electrical activity. • Ambient is noise (room or baby). • Impedance measurements indicate how much the electrical connectivity of the skin is blocked either by dry skin or by debris or oil. There are two impedance measures. The first number displayed is the impedance between the vertex and common sensors, in thousands (k) of ohms. The second number is the impedance between the nape and common sensors, in thousands (k) of ohms. Both impedance measurements must be 12k (12,000) ohms or less before the instrument will be screening. In general the lower the impedance, the faster the test will run. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 3 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE DEFINITION OF TERMS CONT'D: • Interference is caused by two sources, myogenic and/or ambient. When the interference level raises it slows down the testing or stops it. The test may be paused to correct an interfering situation. • Pass is granted when the likelihood ratio is 160 or greater, and the sweep count reaches a minimum of 1000. • Refer is the likelihood ratio in less than 160 after 15,000 sweeps, the screener indicates refer. A refer means further evaluation is needed. UNHS PROCEDURE: Background: 1) UNHS should be conducted by the RN, LPN, or NT on: healthy infants ~ 34 weeks GA in an open crib with normal outer ear anatomy and no obvious deformities of the head or neck. all NICU or ICN graduates ~ 34 weeks corrected gestational age) in an open crib newborns who are not receiving central nervous system (CNS) stimulants newborns whom ideally are asleep or recently fed newborns who are at least 12 hours of age and ideally within 24 hours of age to ensure completion prior to discharge from nursery 2) The newborn hearing screening test should be conducted in the designated area on the mother-baby unit. 3) A brief description of the hearing screening program (UNHSP) should be explained to the parent(s) on admission to the postpartum room. The parent(s) should be given fact sheets regarding the hearing screening test, and an infant developmental checklist. (source = IDPH) Any questions or concerns the parent(s) may have should be addressed prior to the initiation of the test. If the parent(s) declines the hearing screening, the newborn provider should be notified, the parent signs a waiver and the documentation should be placed in the infant's chart. 4) ID band of mother and infant should be checked and matched before separating mother and infant, and upon return of the infant to the mother. 5) Results will be discussed with parent(s) upon return to the room by the person performing the test. In addition, at discharge the newborn provider will discuss the results of the hearing screening test. Selection of newborn: A. Check the Newborn Admission Log Book to obtain a list of infants requiring initial or repeat testing. B. Confer with the nurse caring for the infant prior to conducting the test. C. If the infant is returning from home for an initial or follow-up test, the ABR Newborn Hearing Screening book should be referred to for any significant information. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 4 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE Setting up for screening and techniques of screening: A. Check availability of supplies: 1. Two ear couplers 2. Three jelly tabs 3. Nu-Prep prepping pads, NuPrep gel, and/or other prepping materials. B. Turn on power Press the power switch, which is located at the right side of the ALGO screener. (A selfdiagnostic test will run. Do not touch the keypad or until after this process is completed. After complete, the power light will remain lit.) C. View Main Menu The main menu display appears on the display screen after a brief series of start-up messages are completed. D. Enter infant information E. Determining Test Mode The main menu display provides four modes of testing: 1. Simultaneous screen mode- tests right and left ear at the same time. 2. Sequential mode- tests right ear and then left ear, one after another 3. Single ear-right- tests right ear only 4. Single ear-Ieft- tests left ear only The simultaneous screen mode is the fastest screening mode for an infant who is deeply asleep, and is recommended for use with well newborn. Sometimes infants are aroused by the simultaneous sound of clicks to both ears, so sequential screening mode (on one ear then the other) would test faster for these infants. Single ear screen mode is used to retest an ear that refers or to test a single ear. Once the screening mode best for an infant has been determined: a. Highlight the mode you want by sliding finger across the touch pad, and click the highlighted word, then press the enter key once. The key can also be used. b. The infant preparation display screen will appear. F. Preparing infant for Screening. 1. Selecting Sensor Sites It is extremely important to place the sensors in one of the two recommended configurations. If the recommended configurations are not used, significant slowing of the test will occur. Standard Placement White (nape) - nape of the neck, midline just below hairline THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 5 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE Green (shoulder) - common place on the shoulder, either side can be used. Black (vertex) - place high on the forehead, midline just below hairline. Warning! An infant's hair should not be cut or the head should not be shaved. Do not use excessive pressure near an infant's fontanel while prepping G. Prepare Sensor Sites 1. Prepping the infant's skin decreases impedance and increases conductivity. By prepping the skin, dry dead skin and surface oils are removed. The prepping techniques provided are recommended for full term, healthy newborns. Special caution should be taken when prepping the skin of premature infants who are now old enough to be tested, but who may still have fragile sensitive skin. Determine skin type, and wash if necessary. For oily, sweaty, or if oil or lotion has been applied to skin, wash each site with mild soap and water. If skin appears normal or dry proceed to the following steps: Nu-prep - wipe a one inch area of skin with Nu-prep using one pad and rotating the pad, all three sites may be prepared. Hold the skin taut and make one to four light strokes across the skin in one direction only. Warning! Excessive force should not be used. Do not use the edge of the pad. Prep using friction not force. If irritation occurs discontinue prepping, and allow skin to recover. Do not break skin. Consider an alternative prepping method before re-prepping. Prepping or sensor application to skin is not to be done if skin is damaged or compromised. Discard after single patient use. 2. Liquid Skin Prep or Gel - Put a small amount of prep (the size of a pencil eraser) on the corner of a gauge sponge. Prep an area slightly larger than the sensor by holding the skin taut and scrubbing the site gently moving the gauze sponge or washcloth. Wait one moment to be sure skin is not very wet. Pat lightly with a gauze or clean cloth if necessary. Warning! Only preps that are safe for infants are to be used. For complete instructions refer prep package insert and follow directions, precautions and warnings. If irritation occurs discontinue use and seek appropriate medical care. H. Placing sensors on infant and checking impedance. 1. Press gently and firmly while placing sensors directly over each prepped site. 2. Attach the Patient Cable Assembly (PCA) to sensors by gently squeezing the legs of a PCA spring clip and place the clip over the end of the sensor tab, then release the leg. Match the cable clip colors to the appropriate sensor site. • Green clip to common sensor (shoulder, cheek, or mastoid). • White clip to nape sensor (back of neck). • Black clip to vertex sensor (high forehead). 3. Observe impedance measurements and determine if impedance level is 12k or less, no further handling of the sensors is required. If impedance level is 12k, gently but firmly press sensor. Re-prep as necessary. A message next to the sensor on the screen will appear. Proceed to Ear Coupler placement. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 6 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE I. Applying Ear Couplers Ear couplers fit around the infant's ear forming a complete seal. 1) Open Ear Couplers at the notch in the corner. 2) Before removing Ear Couplers from the liner attach the colored transducer to the couplets. Only the transducer will be visible inside the Ear Coupler. 3) Remove the first Ear Coupler from the liner card, and place over the entire external ear creating a good seal. (Red tip = right ear, Blue tip = left ear). The cable should be directed toward the top of the infant's head. Do not place pressure over the ear canal or compress the tragus. Apply second Ear Coupler in the same manner. J. Screening . 1. Wait for infant to be quite or asleep 2. Click "Start" 3. Observe test display- All test displays and results displays will reflect your choice of screening mode. 4. Monitor interference occasionally. Test is continuously and regularly being monitored and updated for interference. The sources of interference are myogenic (muscle or electrical) and ambient noise (room or infant). A bar in the middle of the display will light up depending on the degree of interference. This interference may slow the test down. You may pause the test to correct the situation. Resume test after corrections are made. 5. Monitor impedance occasionally. Observe the display periodically for impedance. Every 500 sweeps during testing, the impedance measurements are monitored and updated. If impedance levels above 12k ohms are detected at any 500 sweep checkpoint, the impedance light on the front panel lights up indicating the site of the problem, and suspends testing until it is corrected. You will also see the key "word help sensors" light up at the bottom of the display screen. When testing is suspended, the instrument assumes that all sweeps accumulated since the last "500 sweeps checkpoint" were not gathered under optimal circumstances, so the resets the SWP to the last closest 500 sweeps point. The likelihood ratio (LR) remains the same because no more data has been accepted. 6. Observe Results and Print When the testing of both ears are completed, the results are displayed, and the ALGO 5 automatically prints the results. If testing of one ear is completed and the other ear is still being tested, the results of the completed ear can be printed, a copy can be obtained by using the touch tab to highlight the print keyword and click enter once. 7. Clean Up and Shutdown a. Remove the spring clips from the sensors by pinching the clip "legs". b. Remove the acoustic transducers from the ear couplers. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 7 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE c. Put the ATA and PCA assemblies in the drawer beneath the workstation. d. Remove sensors and ear couplers from the skin, carefully supporting the underlining skin with your fingers, and lifting the edges of the sensors gently. If the sensors need assistance to remove, use water on gauze or cotton swab to soak and soften hydrogel along the sensor edges. Warning! Do not leave sensors on infant for a prolonged period of time. Irritation may develop if sweating occurs. e. Remove any residue left by the sensors or prepping materials by wiping each sensor site with clean water and gauze. f. Dispose of single-use patient items by putting them in the trash can. g. When screening for the day is complete, turn off the instrument off. CAUTION! Be sure to turn the power off before disconnecting the keyboard, ATA, or PCA to prevent system failure. If the patient cable assembly is not stored correctly this can result in damage to the ALGO5 from static charge NURSING OBSERVATION: Observe the infant for any changes in status during testing, and notify the medical provider immediately. DOCUMENTATION 1. Document the results in the Newborn Admission Book and the newborn electronic medical record. 2. Apply the result notification to the infant's medical chart. PROCEDURE FOR HANDLING REFER #1 RESULTS A retest is conducted only on the ear with REFER before discharge and prior to scheduling the baby for an audiology follow-up appointment. o If the test result is a PASS, the tester informs the parent(s) of the initial false positive result from the first testing and that the newborn's hearing is normal. In addition, the newborn provider will discuss the UNHS results at the time of discharge. o If the test result is REFER again (#2) o The tester will notify the RN caring for the baby. o RN notifies the newborn medical provider who will discuss the UNHS result with the parent(s). o Newborn refer after retesting will be documented in powerchart which will trigger automatic referral to the dept of Audiology. o Medical Provider will inform parents of result and given the phone number of audiology department to make follow up apt. Audiology Dept. is 312-996-6520. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 8 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE HI TRACK Data Management System Department data analyst completes the following information All Live Births are entered into HI Track Data Management System by Department . Twice weekly all information from ALGO 5 Hearing Screener is downloaded into Hi Track Data Management software. Information is reviewed and verified for accuracy in the screening area of HI Track. File is transfer to the State thru the HI Track system twice weekly. Monthly review of data to ensure accuracy and information matched with Hospital record of live births. ACTION TAKEN BY UIC AUDIOLOGY Newborns referred to audiology for REFER results will be retested in the outpatient audiology clinic. Newborns who fail their hearing re-evaluation will undergo a comprehensive evaluation depending on unilateral versus bilateral hearing deficit and infant age (UIC Audiology failed UNHS evaluation guidelines). The goal of this program is to correctly identify all hearing impairments in infants by three months of age with the institution of an intervention process by 6 months of age. All failed hearing evaluations are reported to IDPH. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 9 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE UIC Universal Newborn Hearing Screening Guidelines UNHS Completed PASS REFER #1 Repeat UNHS Completed PASS REFER #2 Action Taken by 4 West -Newborn refer after retesting will be documented in powerchart which will trigger automatic referral to the dept of Audiology. -Medical Provider will inform parents of result and given the phone number of audiology department to make follow up apt. Audiology Dept. is 312-996-6520 Outpatient Audiology Testing PASS FAIL Audiology failed UNHS evaluation guidelines Action Taken By UIC Audiology: 1. Phone call to parent 2. Letter sent to parent 3. Notify IDPH after second failed appointment THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Women & Children’s Health Services DATE: 11/07 PAGE 10 of 11 Reviewed 12/10 UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO CLINICAL CARE GUIDELINE REFERENCES 1. Herrmann, Barbara S. Auditory Neuropathy and Universal Newborn Hearing Screening, NATUS Medical 2008. 2. White K, Vohr B., Behrens T. (1993) Universal newborn hearing screening using transient evoked otoacoustic emissions: Results of the Rhode Island Hearing Assessment Project. Seminars in Hearing, 14(1): 18-29. February. 3. Wrighton, Steven, MD. University of Kentucky. America Family Physician 2007. May 1, 1975 (g): 1349-1352. Universal Newborn Hearing Screening. 4. Stone, Kurt A. MD, Smith, Brian D., Lembke, Jeania M MD, Clark, Leroy A, MD, McLellon, MaryBeth RN BSN, May 9, 2000. Original Research. Universal Hearing Screening. 5. Stein L. et al. (19910). The effectiveness of screening programs based on high-risk characteristics in early identification of hearing impairment. Ear and Hearing, 12(5). 6. Jacobson J. et al. (1990) Automated and conventional ABR screening techniques in high-risk infants. Journal of American Academy of Audiology, 1(4): 187-195. 7. Natus Medical Inc., Newborn Hearing screener ALGO 5- User Manual, 2008. 8. Murray G. Ormson MC. Et al. Evaluation of the Natus ALGO 3 Newborn Hearing Screener. J Obstet Gynecol Neonatal Nurs, March 1, 2004; 33(2): 183-190. 9. Year 2000 Position Statement Principles and Guidelines for Early Hearing Detection and Intervention Programs. Pediatrics vol 106(4); October 2000, 798-817. 10. Thompson DC. McPhillips H. et al. Universal Newborn Hearing Screening: Summary of Evidence. JAMA, October 24, 2001; 286 (16):2000-2010. THE UNIVERSITY OF ILLINOIS MEDICAL CENTER AT CHICAGO Nursing Services Women & Children’s Health Services DATE: 11/95 PAGE 11 of 11 Reviewed 12/10 ________________________________ Krystal Revai, MD, MPH Director of Newborn Services Associate Professor of Pediatrics ________________________________ Beena Peters, MS, RN Associate Director Women & Children’s Health Services ________________________________ Date ________________________________ Date ________________________________ Diana Tirol, BSN, RN Nurse Manager, Mother/Baby APSD Women & Children’s Health Service ________________________________ Date