Download C`ENTER
Transcript
Umted States Patent [191
[11]
Patent Number:
Mezei, Louis M. et a1. '
[45]
Date of Patent:
[54]
USER CONTROLLED OFF-CENTER LIGHT
4,253,846
4,255,788 3/1981 Schwartz et al.
364/413.07
. . . ..
LING AND REACI'ION
[75]
Inventors: Mezei, Louis M_, Fremont; Bradley
‘
4,268,268
5/1981
Blum
4,319,882
3/ 1982
Sharma ........... ..
. .... .. ..
4,451,433
5/1984 Yamashita et a1.
422/63
4,478,094 10/1984 Solomaa et al. ..
73/ 863.32
4,556,641 12/1985 Kano et al.
Stephen J. Moehle’ both of Berkeley;
4,580,895
4/1986
Patel . . . . . . . . . . . . . .
436/52
436/63
422/73 x
. . . .. 356/39
Brent S. Noorda,
Pleasant H111,
.
4, 665 , 553 5 /l987
4,608,246
8/1986
Bayer et a1.
Gershman et al.
.. 424/11
356/39 X
J°sePh T- W‘d‘mas’ Berkeley; James
A- Zei?in, P‘edm‘mt’ all 01' Cahf-
4 678 894
4,683,120
7/1987 Shafer ............. ..
7/1987 Meserol et a1.
364/416 x
....... .. 422/72
.
'
'
'
4,713,348
Asslgnee. Cetus Corporation, Emeryvllle, Cahf.
Appl. No.: 906,101
[22] Filed:
Smythe et a1. ...................... .. 436/53
s_ Albom, Richmond; coppock, Stan;
- ,
[73]
[21]
Oct. 10, 1989
ABSORBANCE READING ADJUSTER IN A
AND
3/1981
4,873,633 .
12/1987
Ullman ........ ..
436/501
4,719,087 V1988 Hanaway .... n
422/102
4,727,033 2/1988 Hijikata et a1. ..................... .. 356/39
Sep. 11, 1986
Primary Examiner-Clark A. Jablon
Attorney, Agent, or Firm-Ronald C. Fish; Kevin R.
Kaster; Albert P. Halluin
[57]
ABSTRACT
A host computer controls a plate reader which optically
Related US. Application Data
[63]
Continuation-impart of Ser. No. 788,998, Oct. 18, 1985,
abandoned’
[51]
Int. Cl.4 ................... .. G01N 33/48; GOlN 21/01;
reads the results between donor samples and reagents.
G01N 33/80; G01N 35/02
The system provides the user with the ability to change
[52] US. Cl. ............................... .. 364/413.08; 356/39;
356/442; 422/73
[58] Field of Search ---------------- -- 364/416, 555; 422/73;
356/39, 4421 440
[56]
References Cited
the position of off-center light absorbance readings to
maximize the power of the machine to discriminate
between positive and negative agglutination reactions.
In addition, the user can tailor thresholds for absorb
ances used to distinguish between positive, negative and
“no type determined” reactions.
U.S. PATENT DOCUMENTS
4,130,395 12/1978 Chryssanthou ..................... .. 424/11
7 Claims, 4 Drawing Sheets
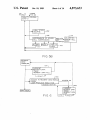
W
HAVl
C
ABSORB
ANC E
POSITIVE
REACTIONS
1,511
‘POSITIVE
ACTUAL ABSORBANCE
AT POSITIVE THRESHOLD
.1 1 1,.
NEGATNE
NEGATlVE
THRESHOLD ‘maesuow REACTIONS Atrgls’gll-?
ACTUAL ABSORBANCE
AT NEGM’NETt-RESHOlD
ABSORB
ANCE
POSITIVE
REACTION
AGGLUTINA
TION
NUMER OF NTD'S
AT CURRENT
THRESHOLD
SETTING
WELL
C‘ENTER
""11 \
meant?“
WALL
‘~—'
U
DEFINA
READINBéE
POSITIONS
131141958811“
WELL WALL
US. Patent
HOST
0ct.10,1989
Sheet 1 0f 14
[/20
4,873,633
MAINFRAME
CPU
DEC
PDP
II
30
PRINTER
/
(32
(22
KEYBOARD
28
CENTRIFUGE
(
PLATE
READER
TERMINAL
24J
ORBITAL /34
SHAKER
I
LIQUID
HANDLER
26
LIQUID
HANDLER
AND PLATE
#2
HANDLING
MECHANISM
FIG. I
LIQUID
HANDLER
. . .
#N
US. Patent
Oct.10,1989
Sheet 2 of 14
4,873,633
US. Patent
Oct. 10,1989
I Sheet 3 0114
4,873,633
LOGON FOR SYSTEM
STARTUP USER
_/lO2
PRIV TABLE FIVE
MAIN MENU
#104
LOG ON FOR
SYSTEM sTARTUP
REAGENT DATA
HANDLING
"54
('40
QUALITY CONTROL
PROCEDURES
LIQUID HANDLING
PARAMETER
A00
DEFINITION
PLATE READING
EDIT RESULTS _/-|7O
(I90
REsULTs
SUMMARY
READER
CALIBRATION
/— O0
5
( 300
SYSTEM
CONFIGURATION
FILE
OPERATIONS "400
.
('38
IM MUNOASSAY
F IG . 3
US. Patent
Oct. 10, 1989
Sheet 4 0f 14
4,873,633
LIQUID HANDLING
MENU
A06
(H2
'
BROM PROCEDURE FOR
3 ABO/Rh TESTING:
'08
[H6
PROCEDURE FOR
ABO/Rh TESTING=
SAMPLE HANDLING
REAGENT HANDLING
QROCM PROCEDURE FOR
no,
ABO/Rh TESTING ,-II4
L
SELECT CRITERIA "'8
FOR REPEAT LIQ.
HANDLING
I
(120
(I30
OPTIONS F OR
SPECIFYING WBN'S
NTD S
OF SAMPLES FOR
I
REPEAT LIQ.
HANDLING
[T122
‘
DISPLAY OF NTD'S
—‘
PROCESSED BY WBN
_
I
PRINT
(I32
PROC. FOR REPEAT
LIO. HANDLING
Q__l
FOR SAMPLES
PROC. FOR REPEAT A24
LIQ. HANDLING
_
('34
FOR NTD S
I
B
BROM PROC. FOR REPEAT
YES LIO. HANDLING
(
126
PROC. FOR REPEAT
YFEOQA LIO. HANDLING OF
NTD'S= SAMPLE
(I28
_
SAMEEZAN‘éE'PINE
HANDLING
FOR SAMPLES:
SAMPLE HANDLING
('36
SAME: REAGENT
HANDLING
i
FIG. 4
US. Patent
Oct. 10, 1989
Sheet 5 of 14
4,873,633
QUALITY
CONTROL
MENU
NO BROMELIN PROCEDURE FOR202
PRETREAT
CHECKING REAGENT
CELL SUSRENSIONS
(I64
(204
ABSORB OF REAGENT
BROMFEEQ QEIXEEIEQRCCETECSISSNS
CELL SUSP
FRET
A
SAMPLE HANDLING ‘
TABLE a MENU
RESULTS NOT
ACCEPU[ ] VIEW
I N67
ACCEPTED
(#68PROC. FOR CHECKING
(206
QC
ADJUST CELL
PRINT
CHART
»
SUSPENSION
REAGENT CELL SUSF;
J
REAGENT HANDLING
L
DO QC LIQ.
|
HANDLING
BI
PLATE READING
.
QC OPERATOR OK
ID LOGON
NO
LIO. HANDLING
ID OF QC BROM
SAMPLES
I18; _I LI42
LI44
(‘I46
SIEOCEDURE FOR
—
BROM FROG FOR QC;
YES
SAMPLE HANDLING
RESULTS OF
Qc PROcEDURE /-'52
I48/
I-IEJ
PROC. FOR QCI
REAGENT HANDLING
PERFORM QC
LIQ. HANDLING
150/
"'53
L
a cOLLEcT
~
ABS. DATA
NO
BROM PROG. FOR H69
CHECKING
DONOR CELL
SUSP
1
fl?!
PROc. FOR
BRQM CHECKING
I75\
DO QC LIQ
HANDLING 'a
fl73
PROc. ‘FOR
CHECKING
DONOR GELL
DONOR CELL
E‘IISNPISLSIRIIPLE
RIEESEEN‘TSIS’IIN‘DLING
I
PLATE READING
CHECKING REAGENT
IGIJ
PROc. FOR SAMPLE
EAIIEIINGFOR
IrERGO
To
EICHEER
REAGENT TITER
_—____I
58
FIGSB
HANDLING \IGT
~—l63
FIG. 5A
US. Patent
To
FIGSAT
0ct.10,1989
Sheet 6 of 14
4,873,633
FROM
‘FIG. 5A
QUALITY CoNTRoL
CoNT' D
1
VIEW TITER
GRAPHS
'62
I
(I66
(‘I70
ABSORBANCE OF DoNoR
CELL sUsPENsIoNs
‘
‘
RESULTS
NOT
ADJUSTING
DONOR CELI
‘ ACCEPTED SUSP
ACCERTI [PRINT )—1 CHART
VIEW (QC/I75
77
I
REAGENT
DATA
HANDLING
I56w
CURRENT
REAGENT DATA
TABLE
REAGENT DATA FILE
l— PRINT J
('58
ACCESS To REAGENT DATA MODULE
LOGON
UsER RRIvILEGE TABLE FIVE
LACCESS NoT OK
ACCESS OK
(I60
CURRENT
REAGENT DATA
TABLE
REAGENT DATA
FILE
[MAIN MENU‘
F166
EB LPRINTJ
US. Patent
0a. 10, 1989
Sheet 7 0f 14
4,873,633
PLATE READING
MAIN MENU
EDIT RESULTS
REREAD ROW
fws
AUTOMATICALLY
I
LOGON TO ENTER
OR EDIT ABO/Rh
TEST RESULTS
MANUALLY
NO ACCESS |_
ACCESS
OK
ENTER 0R
EDIT
RESULTS
MANUALLY
'
ENTER
RESULTS
MANUALLY
FOR WBN
I
EDIT RESULTS
FOR WBN
LOGON TO ENTER
OR EDIT DU TEST
RESULTS
MANUALLY
/I72
ACCESS
OK
ENTER OR
r-I74
EDIT DU
RESULTS FROM
CURRENT LIST
No ACCESS I=
LOGON TO ENTER
OR EDIT MISC.
TEST RESULTS
M A NUALLY
_
NOACCESS
L
I
SELECT
ACCESS TEST TO
OK
ENTER EDIT
RESULTS
I
I
ENTER OR
EDIT RESULTS
FOR SELECTED
TEST
l
ENTER REMARKS
MAIN MENU
FIG. 7
US. Patent
Oct. 10,1989
4,873,633
Sheet 8 of 14
RESULTS SUMMARY
(2H
WBN LIST FOR
SPECIFIED
DISPLAY OF WBN “20'
T" GROUP AND
TEST RESULTS
TYPE
I
(2I3
DISPLAY OF
T’ TEST STATUS
207
@ (215
DIRECTORY OF
,
" PLATE INFO TABLE
EEEIJ
DISPLAY OF
UNVERIFIED Rh
NEGATIVE LIST
E
FOR PLATE 4?
_
I
j
DIRECTORY OF
ANTI BODY SCREEN
PLATES
POSITIONS FOR
EZIT
l
PLATE =I=I=
E
DISPLAY OF
ABO/ Rh TOTALS
DISPLAY OF DATA J
__|93
~223
E
(225
LIST OF OMITTED
CAROUSEL
F POSITIONS
_.IPRINT LAB TEST RECORD
E
FIG. 8
US. Patent
4,873,633
‘Sheet 9 0f 14
Oct. 10, 1989
FIG. 9A
502
PLATE READER
NO
CALIBRATION LOGON‘ Acct-35
READER CALIBRATION _,_ 504
MENU
@
( 506
—-| DETERMINING THRESHOLD VALUES I
(“508
BROM PROC. FOR LIQ. HANDLING
OF THRESH. DETERM PLATES
NO
r510
BROM PROC, FOR SAMP HANDLING
YES FOR THRESH. DETERM. PLATES
PROC. FOR REAGENT HANDLIMS
. OF THRESHOLD DETERM PLATES
I
SPECIFY HISTOGRAM
TO
REVIEW
_
.
I
EVAL. TEMP THRESHOLD
VALUES MENU
MANUAL ENTRY OF SAMPLE
INFO FOR EVAL OF TEMP
THRESH. VALUES
L
DISPLAY OF NTDS DERIVED
FROM THRESH. DETERM.
PROCESS
I
NOT OK
DIS P LAY OF DISCRE PANCIES
BETWEEN ENTERED SAMPLE
VALUES AND THRESHOLD
2K
DETERM. PROCESS
520i
522A VERIFY UPDATE OF
F— THRESHOLD
NO
TO
r98.
FROM
FIG. 9B
VALUES
REPLACE
I
I
REPLACE REVIS E
H I STOGRAMS
524
532
L___________I
OK
INoT OK (528
SPECIFY HISTO
GRAMS TO REVIEW
I
( 5530
REVIEW OF EDITED
THRESHOLD
VALUES MENU
| {2 3 4 5
US. Patent
TO FIG.
9A
Oct. 10,1989
Sheet 10 0f 14
4,873,633
FROM
FIG. 9A
I
{540
BROM PROCEDURE FOR
PLATE READING
YES POSITION
DETERMINATION
f544
SAME- REAGENT HANDLING
(542
RRoc. FOR PLATE
BROM READING POSITION
NO
DETERMINATION:
LIQ. HANDLING
V
(548
STEPS OFF CENTER
DETERMINATION
L546
(554
VERIFY UPDATE OF
PLATE READING
POSITION
FIG. 9B
US. Patent
L06 0 N
0a. 10, 1989
NO
ACCESS
Sheet 11 0f 14
4,873,633
MAIN MENU
FILE OPERATIONS
MENU
TRANSFER COMPLETED
r404
RESULTS TO DISK
408
_ REVISE STORED
DATA
I
RESET DATA
FOR SPECIFIED
WBN ENTRY
‘DELETE DATA lI
RESET DATA
FOR SPECIFIED
PLATE ENTRY
4IO
IL——I
DELETE ENTRIES DELETE ENTRIES DELETE
BY DATE
BY PLATE
BY WBN
K
I
k
4II
BACKUP FILES
4I2
406
DISPLAY DATA STATISTICS
FORMAT FLOPPY DISK/—-402
MAIN MENU
FIG. IO
(
4I3
DELETE IF
TRANSFERRED
TO MAINFRAME
IL4I4
US. Patent
Oct. 10, 1989
Sheet 12 Of 14
4,873,633
NO
ILO GON F OR SYST EM c ONFIGURATIONI'——>@
ACCESS
ACCESS OK
SYSTEM CONFIG.
MENU
(304
(306
MODIFY CURRENT
TEST FORMAT
MODIFY ADO/Rh
LIQ. HANDLING
PROC.
I
I
@
(‘302
CURRENT TABLE OF
USER ID/ SYSTEM
SECURITY INFO
I
(30s
—-IMODI FY MAINFRAME HANDSHAKE]
3270
EMULATION
SNA
EMULATION
SPECIFY HANDSHAKE
FOR RS232 PROTOCOL
I
I
I
—-IsEI_EcT LAB REPORT FORM |~3IO
J
MODIFY IMMUNOASSAY FORMAT _,3|4
AN D LIO. HANDLING
I
_-IMODIFY ERROR HANDLING METHODI~3I2
I
—-IMODI FY HARDWARE OOMPLEMENT]
I
FIG. II
US. Patent
Oct. 10, 1989
Sheet 13 0f 14
4,873,633
0/0 OF
TOTAL
wELLS
HAVING
EAcH
ABSORB
ANCE
I
I III II PPM“ II
WES‘?
POSITIVE
5'9
yPOSITIVE
REACTIONS
xxxx’TfL23
I II
¥_“’—/
NEGATIVE
NEGATIVE
THRESHOLD THRESHOLD REACTIONS
YYYY/ 525
ACTUAL ABSORBANCE
AT POSITIVE THRESHOLD
ASAIVIRLE
W
POSSIBLE
22 2’/5.2 I
ACTUAL ABSORBANCE
AT NEGATIVE THRESHOLD
NUMBER OF NTD'S
AT CURRENT
THRESHOLD
SETTING
WELL
ABSORBANCE
CENTER
POSITIVE
REACTION
AGGLUTINA
TION
I
POS TION
ABSORBANCE READING
POSITIONS
FIG. ISA
WALL
READING
I
RELATIVE TO
H4
WELL WALL
USER
DEFINABLE
READING
POSITIONS
FIG. I38
US. Patent
Oct.10,1989
Sheet 14 0f 14
4,873,633
ABSORBANCE
I<——|65
I
|
l
|
I
I
:
2
:
4
:
8
|
'
: i
I6 32
DTLuTToN OR TITER
ABSORBANCE
MAX.
,
.
_
- ' ' "
MEAN
MIN
I23
WBN
I23
135
620
3o---- eo---
90
DAY
KNOWN
EXPERIMENTALLY
WBN
TYPE
DETERMINED
BLOOD TYPE 23 TEMPLATE
FAPOS
BPOS
o
BPOS++———++
APos +——+++—+'
o
FIG. l6
— —
+ —
— —
+
+
1
4,873,633
2
not subject to automated liquid handling. Test results
USER CONTROLLED OFF-CENTER LIGHT
ABSORBANCE READING ADJUSTER IN A
LIQUID HANDLING AND REACTION SYSTEM
This application is a continuation-in-part of Ser. No.
788,998, ?led Oct. 18, 1985, now abandoned.
CROSS REFERENCE TO MICROFICHE
APPENDIX
There is included herewith micro?che appendices,
consisting of 10 micro?che and 461 frames, including
the hex format object code controlling the various pro
from these antibody screen tests and other tests done by
hand must be recorded for each donor’s blood for
which these tests are done. Further, testing procedures
for blood typing can change over time in that different
dilution values are often needed for different batches of
reagents to get the proper absorbance readings. Fur~
ther, the amount of dilution of the red blood cells
should be optimized. The data for the optimal dilution
values must be recorded.
Further, other process parameters of the blood typing
sequence should be subject to customization to enable
the various users to customize their testing procedures
to conform to local practice. This data de?ning the
cessors in the system.
15 characteristics of each step, such as the amount of each
BACKGROUND OF THE INVENTION
sample to be placed in each well, which wells in which
to place samples, the amount of dilution in certain steps,
The invention pertains to the ?eld of systems for
the wells to place reagents in, the number of mixes at
automatically performing blood typing operations, and,
various points in the process and various other criteria,
more particularly, to systems for controlling automated
liquid handling apparatus to do blood typing and for
should be subject to customization, should be easily
interpreting the results from a plate reader and for man
changeable, and should be remembered by some mecha
aging the data generated by the liquid handling appara
nism so that it can be automatically invoked each time a
tus and the plate reader apparatus and for printing vari
procedure is performed without having to look it up
every time. Of particular importance is the amount of
dilution of each particular reagent which should be used
ous reports.
Modern blood banks must perform thousands of 25
blood typing and antibody screening operations and
manage the data resulting from such tests. These opera
to optimize the absorbance for that reagent and to con
serve the amount of reagent used. These reagents are
tions involve the handling of thousands of samples of
donor blood, the pipetting of reagents into samples of
often quite expensive.
the plasma and red blood cells from the donor blood
accountability system such the quality of data generated
in the testing is consistently high and the persons per
forming the testing can be determined. For example, it
and the optical reading of the wells containing the
donor samples and the reagents to determining the pat
Further, it is useful to have a quality control and
is useful to know the expiration data for all the reagents
in stock and the associated absorbance values for each
constitutes a template which characterize the blood as 35 reagent lot over the period of its usage. It is also useful
to compare the test results for known sample types to
being from a particular blood group and having a par
the results that should have been obtained as a check on
ticular Rh factor. Each test of each donor’s blood in
the accuracy of the system. It is also useful to be able to
volves pipetting of multiple samples of that donor’s
generate reports on daily or monthly activities to deter
plasmas into multiple wells and pipetting of multiple
mine the amount of certain types of blood in stock and
samples of diluted red blood cell samples into multiple
where it can be found. The number of “no type deter
wells. Multiple diluted reagents are then added to the
multiple wells containing plasma and red blood samples
mined” test outcomes (hereafter NTD).
Thus, a large amount of data in the form of process
and various reactions either occur or do not occur de
controlling parameters and test results are involved in
pending upon the blood type and the reagent in each
well. Typically, these reactions manifest themselves as 45 blood typing and antibody screening operations, and a
large amount of record keeping for this data is involved.
clumps of protein in the bottom of the well for a posi
Therefore a need has arisen for a system which can
tive reaction and no clumps for negative reactions, the
tern of positive and negative responses to various rea
gents. The pattern of positive and negative responses
pattern of positive and negative reactions determines
perform these thousands of liquid handling steps reli
the blood type.
The blood type can be determined by placing the
multiple wells under a strong light source and reading
ably and tirelessly and which can handle the thousands
the optical absorbence, i.e., the amount of light which
gets through the bottom center of the well. Typically
absorbance readings are taken on both sides of the cen
of data records which characterize the liquid handling
and which constitute the data record for each donor.
Further, such a system should be access controlled and
implement accountability. It should also ease quality
control operations and maintain data useful in quality
ter, bottom of each well, and the results are compared 55 control efforts. Such a machine should also maintain all
test results and be able to communicate them to a main
to certain threshold criteria for absorbance. The com
frame computer for permanent storage or allow them to
parison of the readings will indicate the presence of a
be archived onto permanent magnetic storage. Such a
clump at the bottom of the well (low absorbance in the
machine should also enable the automated generation of
off center readings) and therefore a positive reaction or
the absence of a clump and therefore a negative reaction 60 reports for management.
(high absorbance on both off center readings).
SUMMARY OF THE INVENTION
Clearly, the process of blood typing of thousands of
The invention is a system for performing a host of
donor samples involves many thousands of liquid .han
functions which aid in the operational work and man
dling steps and the generation of many thousands of
absorbance readings for the multiple wells devoted to 65 agement of a blood bank. The invention consists of
various off the shelf components and a custom designed
each donor. Further liquid handling steps are involved
liquid handler combined in one system, all controlled by
in automated antibody screening, and some special tests
may have to be done by hand on the blood which are
a comprehensive software resident in a host computer
3
4,873,633
4
which allows the user to control blood typing and other
host is also coupled to an orbital shaker and a centrifuge
liquid handling operations in the liquid handler and to
for re-suspension and re-separation of liquids and sam
ple cells during some system operations.
manage the data which results therefrom. The system
automates: much of the liquid handling involved in
The host software also contains routines for imple
processing donor samples for ABO/Rh blood typing
tests and antibody screening; sample and plate identi?
menting quality control operations. Such operations
include verifying test procedures by processing a small
number of known blood type samples and checking the
experimental data against the known results, and verify
ing that the dilution of cell suspension reagents and
donor cells produce absorbances in the proper range.
The system can also test the titer of antibody reagents.
The data management functions of the invention
cation using a bar code reader; plate reading for the
ABO/Rh tests; record keeping for quality control data
and test results for individual samples; access to infor
mation on test status; and printing of laboratory records
and transmission of information to a mainframe com
puter for on-line storage.
The physical system includes a Digital Equipment
Corporation PDP-ll MICRO with a 10 megabyte hard
include the management of data in the form of: test
procedure parameters for liquid handling, plate reading
disk and a VT220 terminal. The host system 20 runs a
commercially available operating system. The control
software is resident in the host. It is coupled to a custom
designed liquid handling system which has several mi
croprocessors resident therein which control various
stepper motors, bar code read heads and monitor vari 20
ous sensors. The process controlling parameters to con
and histograms; quality control data consisting of rea
gent lot numbers and expiration dates with associated
absorbances of these reagent lots over the period of
their usage; test results including blood groups and
probable type; manually added data from such tests as
DU tests, antibody screens, hepatitis, HTLV III, CMV,
RPR and other tests; and test status for the day of a
trol operations of the liquid handler come from the host
range of parameters such as the number of A+ samples
computer after being de?ned by the user at the host
that have been processed, the number of NTD’s during
terminal. Once the process parameters are loaded, the
resident software in the liquid handler controls various 25 the day and the status of testing for particular plates.
transfers of donor blood plasma and red blood cells to
BRIEF DESCRIPTION OF THE DRAWINGS
various wells in plates loaded by an automated plate
FIG.
1 is a block diagram of the interconnection of
reader. The resident software also controls dilution of
the various components of the system.
the red blood cells before depositing them in wells and
FIG. 2 is perspective view of the liquid handler of the
the transfer of speci?ed quantities of various types of
system.
reagents into the wells containing the donor’s blood
FIG. 3 is a flow diagram of the user options for vec
plasma and diluted red blood cells. Each plate can hold
toring processing to various sub-functions that the sys
samples from eight donors, and the donor samples are
tem can perform.
stored in test tubes in a circular, rotating lazy-susan-like
FIG. 4 is a flow diagram for steps performed in al
device which is controlled by the software of the liquid 35
lowing the user to customize the process parameters
handler.
The plates so ?lled are then unloaded by a plate han
controlling liquid handling operations.
dler device, and manually transferred to a plate reader
FIGS. 5A and 5B are a ?ow diagram of the process of
Model Autoreader EL309 manufactured by Biotech
allowing the user to monitor and control various quality
Instruments which is also coupled to the host. The plate 40 control functions.
reader then obtains absorbance readings on the wells in
FIG. 6 is flow diagram for the process of allowing the
the plates after reading the bar codes on each plate.
user to create and access various reagent data for use in
These absorbance readings for each well on each plate
insuring proper processing and improving reliability of
are sent to the host with the bar code of the plate for
test results.
45
interpretation.
FIG. 7 is a flow diagram of the plate reading function
The host knows which wells of each plate contain
of the system and a flow diagram of the process fol
samples from which donors having obtained this infor
lowed in allowing the user to edit or enter various re
mation by downloading the bar code data read by the
sults from testing.
liquid handler from the donor test tubes and the plates
FIG. 8 is a ?ow diagram for the process followed in
that received samples from each tube. The absorbance
allowing the user to specify results summaries the user
values are interpreted by plotting histograms for the
number of samples having each absorbance for each
would like to see.
FIGS. 9A and 9B are a ?ow diagram of the process
that the system follows in allowing the user to calibrate
different type of reagent. The user is then allowed to
de?ne the threshold values between the positive and
negative reactions for each reagent by viewing the
histogram and graphically moving an arrow to the de
sired threshold value. A “no man’s land” region where
the absorbance does not clearly indicate either a posi
tive or a negative value is also de?ned, and samples
having absorbance values falling in this region are
tagged as NTD. The user de?ned thresholds for each
type of reagent de?ne “templates” or patterns of posi
55
the plate reader and to adjust the thresholds used by the
host computer in interpreting test results.
FIG. 10 is a ?ow diagram of the process used by the
system in performing various ?le operations.
FIG. 11 is a flow diagram of the process used by the
system in allowing the user to con?gure the system for
a speci?c installation.
FIG. 12 is an illustrative histogram for a single rea
gent illustrating the meaning of adjusting the thresholds
tive and negative reactions which will de?ne each par
used in interpreting test results.
ticular blood type. The absorbance values for each
FIG. 13A shows a typical well bottom with a positive
sample are then compared to these templates and typed 65
reaction button.
as to their blood group type.
FIG. 13B shows a typical graph resulting from a
The host is also coupled to a printer, and the user can
request various pre-formatted management reports. The
positive reaction button.
_
5
4,873,633
6
much of the liquid-handling involved in processing
donor samples for ABO/Rh tests and antibody
DETAILED DESCRIPTION OF THE
PREFERRED EMBODIMENT
Referring to FIG. 1 there is shown a block diagram of
screening,
sample and plate identi?cation using a bar code reader,
the system of the invention. The system is comprised of 5 plate reading for ABO/Rh tests,
record keeping for quality control and test results for
a Digital Equipment Corporation PDP-ll MICRO host
individual samples,
computer 20 which coordinates the activities of the
system under the control of the resident software in
cluded herewith as the appendices and the user (not
shown) who enters commands through a keyboard 22 in
response to information displayed by the software on a
access to information to test status, and
printing of laboratory records and transmission of infor
mation to a mainframe computer for on-line storage.
ProGroup Automated Blood Typing System Bene?ts
terminal 24. The host computer 20 runs the commer
The ProGroup TM automated blood typing system is
a ?exible system, designed to adapt to the way work is
cially available Micro RSX operating system for the
PDP 11/23, ll/53 or ll/73 computer versions available
from Digital Equipment Corporation. The application
already organized in the user’s laboratory. The user
de?nes the way he or she wants particular procedures
carried out, and ProGroup TM automated blood typing
software which implements the functions described
herein is included herewith an appendix. The host com
system executes these tasks at command.
puter is coupled by RS232 serial ports and connecting
Automating liquid-handling makes test results more
cables to a number of liquid handling and plate handling
mechanisms of which liquid handler 26 is an example. 20 reliable, and allows employment of lab workers having
less skill. Features like computerized, data management
The preferred embodiment of the system can control up
techniques and the built-in bar code reader reduce the
to 8 liquid handlers. The details of the liquid handlers
incidence of operator error.
and plate handling mechanism are given in a copending
ProGroup TM automated blood typing system does
U.S. pat. application assigned to the assignee hereof
not require any previous experience with automated
liquid-handling equipment, or with computers.
entitled “Automated Liquid Handling Apparatus And
Process With Plate Handler”, ?led Oct. 18, 1985, Ser.
No. 789,945, now abandoned, and another U.S. pat.
ProGroup System Overview
application entitled “Liquid Manipulation Device and
Method”, ?led Jul. 5, 1985, Ser. No. 752,449, now aban 30
doned, both of which are hereby incorporated by refer
ence. The object code for the liquid handler disclosed in
the above identi?ed patent application has been
changed somewhat since the filing date thereof. The
latest object code is included herewith in the appendices
hereto. The object code appendices are labelled for the
particular microprocessor in the liquid handler for
There follows more detail on the individual compo
nents of the system.
Liquid-Handler
5
The liquid-handling portion of the ProGroup TM
automated blood typing system consists of the follow
ing items.
ProGroup TM automated blood typing system liquid
which each is intended.
The host 20 is also coupled by an RS232'link to a
handler 26, shown in more detail in FIG. 2, automates
and an orbital shaker 34 are also part of the system but
are not connected to the host 20. The centrifuge and
four permanently mounted liquid delivery heads (X, Y
orbital shaker are commercially available off the shelf
components and are used to process the plates after the
samples and reagents have been put in the wells by the
liquid handler 26 and before the plates are loaded in the
plate reader 28. Collectively, the above described sys
head 44. One or several of these can be used in the
course of a single procedure, since there is no require—
ment to remove one head to install another. The X, Y
and 63 of several sizes to minimize cross-contamination.
tem may hereafter be referred to as the ProGroup TM
The ProGroup TM automated blood typing system
automated blood typing system or the system.
The ProGroup TM automated blood typing system
automates many aspects of the blood typing and group
111 and a maximum of 200 ul.
the pipetting of donor plasma directly from a carousel
20 of tubes 22 into designated wells of a microplate. The
plate reader 28 which optically reads the absorbance
ProGroup TM automated blood typing system mixes
values for samples in various wells in plates ?lled with
donor red blood cells with diluent in designated propor
samples and reagents loaded from the liquid handler.
tions before transferring the cells to the wells of the
The plate reader is commercially available from BioTek
microtitre plates 56 and 58. ProGroup TM automated
Instruments, Inc. under the designation Autoreader
EL309.
45 blood typing system ?lls the ABO/Rh plate 56 and the
antibody screening plate 58 with the appropriate rea
The host 20 is also coupled by an RS232 link to a
gents from a reagent trough 62. The ProGroup TM
printer 30 upon which the host 20 prints the various
automated blood typing system liquid handler includes
reports of which the system is capable. A centrifuge 32
head 28, 12 channel head 42, ?ll manifold 46, and wash
head and 12 channel head use disposable pipette tips 24
accurately transfers volumes between a minimum of 10
Two identical bar code readers (not shown) are built
ing procedures performed in blood banks. This section 60 into the liquid-handler: one for reading the bar code 21
on the sample tube (actually located above the top sur
face of the carousel 20, and the other for reading the bar
code 57 and 59 on the microplates (actually located on
ProGroup TM Automated Blood Typing System
the other sides of the plates). The bar code on the sam
Functions
65 ple tube is the basis for the Whole Blood Number
(WBN) that identi?es the donor sample. The bar code
The major ProGroup TM. automated blood typing
on the microplate allows the system to pair the test
system functions are broadly de?ned below. ProGroup
results from the plate reader with the WBN of the sam
TM automated blood typing system automates:
provides a brief introduction to the functioning of the
system.
7
4,873,633
8
ple donor. The bar code readers can recognize Code
the computer, so the user can quickly generate hard
Bar or Code 39 labels.
copies of laboratory records.
Plate Stacker (not shown) holds six 96-well micro
plates for ABO/Rh testing. Each plate can accommo
date samples from eight donors. These plates are fed
into the liquid-handler 26 one by one for processing.
Once ?lled, a plate is automatically transferred off the
bed 74 of the liquid-handler, into the other rack of the
The ProGroup TM automated blood typing system is
used by laboratory personnel at three levels:
plate stacker.
The Technician, who operates the machine on a daily
basis, running test and printing results.
7 The Supervisor, who performs quality control proce
dures, adds to or revises test data as necessary, handles
the storage of information on the ProGroup TM auto
‘
mated blood typing system host computer 20, and data
Plate Reader
The Plate Reader 28 reads ABO Rh plates automati
cally and transmits data points to the ProGroup TM
transfers data to a mainframe computer.
The Director, who creates the initial framework for
the ProGroup. This includes tasks such as defining who
will be using the system, how the tests will be carried
out, and how test results should be interpreted. The
automated blood typing system host computer 20 for
interpretation. The results are reported by group and
type, or as NTD (No Type Determined) readings. An
NTD sample can be reread automatically with the plate
Director modi?es this framework as necessary, to re
?ect changes in system users, test protocol, etc..
reader, in which case the results stored on the computer
Security Precautions
are updated automatically. Alternatively, the row of
wells can be examined visually, or the liquid handling 20 The system utilizes a user privilege table and user
identi?cation at logon time to guarantee accountability
and the reading of that sample can be repeated manu
and to insure that only users with the proper authoriza
ally. In the latter case, the user inputs information
tion have access to certain portions of the program and
through the computer keyboard to modify the results of
certain data files.
the sample processing procedure.
The following publicly available documents are in
25
corporated by reference for support material.
Orbital Shaker
MICRO PDP-ll System Owner’s Manual Digital
The ProGroup TM automated blood typing system
Equipment Corporation.
includes an orbital shaker 34 as part of the system. The
Pro/Pette Liquid Handling System User’s Manual
Cetus Corporation.
shaker is used to resuspend the cells after the liquid
handling is complete, and then again after the plate has
been centrifuged. The orbital shaker can accommodate
up to eight plates.
Automated Microplate Reader Model EL309 Opera
tor’s Manual, Bio-Tek Instruments, Inc..
Installing and Using the LASO Printer, Digital Equip
ment Corporation.
Centrifuge
The centrifuge 32 can be an instrument such as the
Beckman TJ-6R Tabletop Centrifuge. It is used in a
known manner on the plates from the liquid handler 26
35
LASO Programmer’s Reference Manual, Digital
Equipment Corporation.
Orbital Shaker User Manual.
ABO/Rh Testing
before they are placed in the liquid handler.
Host Computer
ProGroup TM automated blood typing system auto
mates the typing and grouping of donor samples. This
The host of the ProGroup TM automated blood
typing system is the computer 20, called the host com
puter 20. The host computer 20 directs the operation of
the liquid handler 26 and the plate reader 28, and man
section describes the liquid-handling, resuspension, and
plate reading tasks the ProGroup TM automated blood
ages the data generated by the other equipment. The
user primarily interacts with the ProGroup TM auto
mated blood typing system through the host computer
20. The user uses the host computer 20 to select the
function the user wants the ProGroup TM automated
blood typing system to perform, and to view stored data
in tabular or graphic form. The host computer 20 allows
the user to modify data that has been created through
automatic procedures. The user can also enter addi
tional information pertaining to donor samples, such as
antibody screen results, or Hepatitis and HTLV III
results.
The host computer 20 is a DEC MICRO PDP-ll
computer with a 10 Mbyte hard disk and a VT 220
typing system performs for ABO/Rh testing.
Liquid-Handling Functions
The ProGroup TM automated blood typing system
liquid handler 26 transfers donor plasma out of the tubes
22 into designated wells of a microplate 56. It also trans
fers donor red blood cells into a special predilution
block 60, mixes the cells with a de?ned quantity of
diluent, then pipettes them into the appropriate wells of
the microplate 56. Then the ProGroup TM automated
blood typing system fills the plate with the reagents
from the reagent plate 62 for the assay.
Options
Referring to FIG. 3 there is shown the main menu
and logon process. Referring to FIG. 4 there is shown
the liquid handling process flow chart for the organiza
tion of the software if the liquid handling parameter
terminal with graphics capability. The computer 20 60 definition menu selection 100 is selected in FIG. 3. FIG.
runs the software included herewith as Appendix A on
the RSXll operating system which is commercially
available from Digital Equipment Corporation.
Printer
The ProGroup TM automated blood typing system
also includes an LASO dot matrix printer 30 from Digi
tal Equipment Corporation. The printer is connected to
4 would be reached after the user identified himself in
the logon step 102 and was accepted as a permissible
user. Processing would then vector to the main menu
screen 104 wherein all the options shown in FIG. 3
65 would be presented to the user. If he selected option
100, processing would be vectored to the liquid han
dling menu 106 after going through a logon step (not
shown). The liquid handling menu selection allows the
4,873,633
10
liquid-handler. A set of six 96-well microplates is loaded
into the plate stacker on the other side of the liquid-ham
dler. Each plate can hold sample from eight donors.
user to specify the process parameters of the liquid
handling assay for blood typing, NTD determination
and repeat liquid handling with or without bromelin
pre-treatment. Since these process parameters are criti
cal to accuracy of results, only supervisors or higher are
Reagents for the test, including properly diluted cell
suspension and antisera are placed in a row of wells in
allowed to log into this function of the system. The user
the reagent MicroTrof 62, along with a set of 12 tips.
speci?es the following aspects of liquid-handling for the
This MicroTrof also contains a row of reagents and a
ABO/Rh test:
whether or not a Bromelin pre-treatment is being
row of tips for the antibody screening test to be de
scribed later herein. The reagent MicroTrof is placed at
10 the back of the liquid-handler bed 74.
used, choice 108 or 110,
The predilution block 60 is placed just in front of the
which blood typing test should be executed (the op
reagent Microtrof. The block contains a trough of dilu
tions are A, B, (A,B), Rh, Cell Suspension, A1, A2, B,
ent, and several wells where the donor cells are mixed
and Serm Blank), step 112 and, what test format is being
with diluent.
used (i.e., which tests are being carried out in which
columns of the microplate to de?ne the format and
ABO/Rh Testing Procedure
arrangement of the template, i.e., the pattern of positive
and negative reactions which de?ne the blood type),
When the liquid-handler is set up and ready for oper
ation the ?rst step is to carry out the automatic homing
step 112.
and priming procedure. Then the user pushes the
The liquid handling may be done on the plates in any
START key on the hand-held controller 94. The plate
order and the plates may be read in any order. The
stacker places a 96-well plate on the liquid-handler
liquid handlers such as device 26 read the bar codes of
table, and the bar code 59 on that plate is read. The bar
all the tubes containing donor samples that are pipetted
codes 21 on the ?rst group of eight tubes are also read.
into a particular plate. These bar codes are sent to the
Then the liquid-handler picks up the ?rst ProGroup
host computer 20 along with the bar code of the plate
into which samples from the identified tubes were 25 TM automated blood typing system tip from the carou~
sel and withdraws the appropriate amount of donor
placed. The host computer knows which rows of each
plasma from the top of the tube. Aliquots of plasma are
plate contain aliquots of blood from each donor because
placed, one by one into the wells of the plate 56 that are
the control software of the liquid handler always places
designated for reverse typing tests. Next the liquid-han
samples in the plates in the same order relative to the
dler withdraws the required quantity of donor red
order of the tubes from which the samples were re
blood cells for the forward typing tests. Aliquots of
moved. The identi?cation data of the plates and the
cells are placed into the wells of the predilution block
WBNs that are in each plate are stored by the host
60, which already contain the required amount of dilu
computer in a lookup table along with any tag data that
ent. This diluent had been placed in the wells by the
the liquid handler appends to the identi?cation data for
the plate bar code. Such tag data can include data that 35 microprocessor of the liquid handler previously in ac
the plate is a steps off center plate (this will be explained
cordance with data received from the host regarding
more fully below) or is a donor cell suspension or rea
how much dilution the supervisor wants in the test
procedure for red blood cells. The diluent and cells are
mixed according to data regarding how many mixes
that are desired which was speci?ed by the user and
sent from the host to the liquid handler, and then the
gent cell suspension plate etc. Because this data is per
manently stored in a look up table, the order in which
the plates are read by the plate reader is unimportant.
The plates can be loaded in the plate reader in any
order. The plate reader then reads the bar code for each
plate and sends it to the host computer 20. The host
computer then looks up the bar code in the look up
table, and determines if any special instructions need to 45
tainer (not shown).
When the microplate 56 is ?lled with donor samples,
be sent back to the plate reader, e.g. as to wavelength to
be used for the light or whether or not to do a steps off
the l2-channel head picks up reagents from the reagent
Micro-trof 62 and ?lls the row of the plate 56 containing
the ?rst donor’s samples with the pattern of reagents
center series of light absorbance readings.
Parameters
mixture is pipetted into the forward testing wells of the
microplate. The used ProGroup TM automated blood
typing system tip is then ejected into a used tip con
50
speci?ed by the user in the liquid handling procedure
set-up step previously described (the user must ?ll the
The user de?nes the test protocol by specifying in
reagent trof 62 wells with the proper reagents in accor
steps 114 and 116 for no bromelin and bromelin pre
dance with the desired pattern). The row of Pro/Pette
treatment respectively the:
tips used for this operation is then ejected back into the
volume of donor plasma to be pipetted into the wells
55 Microtrof.
of the microplate,
At this point, the liquid-handling on the plate is ?n
volume of donor cells, the volume of diluent to be
ished. The plate is loaded back into the plate stacker to
mixed with the cells (thus the concentration of the
await completion of the remaining ?ve plates in that
donor cell suspension) and the number of mixes,
group.
concentration of the reagent cell suspension, and
When all six are done, they are moved to the orbital
titer of the reagent antisera.
shaker for resuspension. Next they are centrifuged at
Liquid Handler Set-Up
Bar coded tubes containing properly prepared donor
low speed to cause the cells to settle. Then, they are
resuspended. This sequence of steps disperses the cells
when the test results are negative, but causes the cells in
mated blood typing system carousel. The carousel holds 65 a positive test to clump together in a tight button at the
bottom of the well.
48 tubes and 48 ProGroup TM automated blood typing
blood samples are loaded into the ProGroup TM auto
system disposable tips. The carousel mounts at the side
of the ProGroup TM automated blood typing system
The ?nal step is to load each plate into the automatic
plate reader. Plate reading can occur no less than three